CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Cream-colored or pale brown solid; [HSDB] |
|---|---|
| Color/Form | Fine, cream-colored powder |
| Melting Point | 126-127 °C |
| Solubility | In xylene 41.7-43.5, acetone 167-182, methanol 7.39, ethyl acetate 105-111 (all in g/L, 20 °C) |
| Density | 1.565 at 24 °C |
| Vapor Pressure | 1.2X10-10 mm Hg (1.6X10-5 mPa) at 25 °C |
| LogP | log Kow = 3.49 |
| Kovats Retention Index | 2355 |
| Chemical Classes | Pesticides -> Herbicides, Protox-Inhibiting |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 413.2 g/mol |
|---|---|
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 8 |
| Exact Mass | 412.0204468 g/mol |
| Monoisotopic Mass | 412.0204468 g/mol |
| Topological Polar Surface Area | 62.6 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 480 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
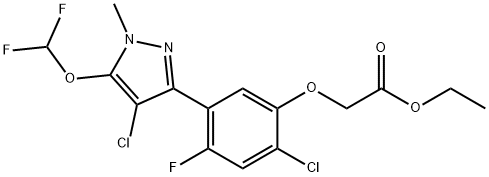
Pyraflufen-ethyl is an ethyl ester resulting from the formal condensation of the carboxy group of pyraflufen with ethanol. A proherbicide for pyraflufen, it is used for the control of broad-leaved weeds and grasses in a variety of crops. It has a role as an EC 1.3.3.4 (protoporphyrinogen oxidase) inhibitor, a proherbicide and an agrochemical. It is a member of pyrazoles, a biaryl, an ethyl ester, an aromatic ether, a member of monochlorobenzenes and a member of monofluorobenzenes. It is functionally related to a pyraflufen.