127779-20-8
Product Name:
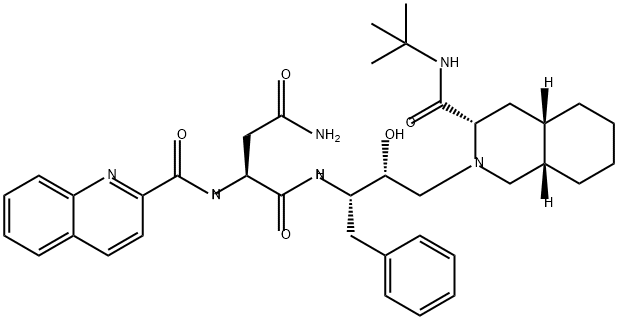
Saquinavir
Formula:
C38H50N6O5
Synonyms:
2-[2-Methyl-3-(trifluoromethyl)phenylamino]nicotinic acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | White crystalline solid |
|---|---|
| Melting Point | 349.84 °C |
| Solubility | Insoluble |
| Vapor Pressure | 2X10-31 mm Hg @ 25 °C /Estimated/ |
| LogP | 3.8 |
| Henry's Law Constant | Henry's Law constant = 9.9X10-35 atm-cu m/mol @ 25 °C /Estimated/ |
| Caco2 Permeability | -6.26 |
| Other Experimental Properties | Hydroxyl radical reaction rate constant = 2.1X10-10 cu cm/molec sec @ 25 °C /Estimated/ |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P405:Store locked up. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 670.8 g/mol |
|---|---|
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 13 |
| Exact Mass | 670.38426872 g/mol |
| Monoisotopic Mass | 670.38426872 g/mol |
| Topological Polar Surface Area | 167 Ų |
| Heavy Atom Count | 49 |
| Formal Charge | 0 |
| Complexity | 1140 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Saquinavir is an aspartic acid derivative obtained by formal condensation of the primary amino group of (2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarbamoyl)octahydroisoquinolin-2(1H)-yl]-3-hydroxy-1-phenylbutan-2-ylamine with the carboxy group of N(2)(-quinolin-2-ylcarbonyl)-L-asparagine. An inhibitor of HIV-1 protease. It has a role as a HIV protease inhibitor and an antiviral drug. It is a member of quinolines and a L-asparagine derivative.