12771-68-5
Product Name:
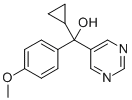
Ancymidol
Formula:
C15H16N2O2
Synonyms:
α-Cyclopropyl-α-(4-methoxyphenyl)-5-pyrimidinemethanol;reducymol;thritone
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid; [Merck Index] White to beige granular crystals; [Reference #1] |
|---|---|
| Vapor Pressure | 0.0000002 [mmHg] |
| Kovats Retention Index | 2187 |
| Chemical Classes | Pesticides -> Plant Growth Regulators |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 256.30 g/mol |
|---|---|
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 256.121177757 g/mol |
| Monoisotopic Mass | 256.121177757 g/mol |
| Topological Polar Surface Area | 55.2 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 295 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ancymidol is a tertiary alcohol that is methanol in which the hydrogens attached to the carbon are replaced by cyclopropyl, p-methoxyphenyl and pyrimidin-5-yl groups. By inhibiting gibberellin biosynthesis, ancymidol reduces plant growth, resulting in reduced internode elongation and thus more compact plants. It is used in the commercial production of a wide variety of container-grown bedding and foliage plants, including chrysanthemums, Easter lilies and poinsettias. It has a role as a plant growth retardant, a cellulose synthesis inhibitor and a gibberellin biosynthesis inhibitor. It is a tertiary alcohol and a member of pyrimidines.
