126-11-4
Product Name:
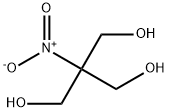
Tris(hydroxymethyl)nitromethane
Formula:
C4H9NO5
Synonyms:
2-Hydroxymethyl-2-nitro-1,3-propanediol
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Liquid |
|---|---|
| Color/Form | Crystals from ethyl acetate and benzene |
| Odor | Odorless |
| Melting Point | 214 °C (pure) |
| Solubility | Soluble in polar solvents such as methanol and isopropanol. Insoluble in non-polar solvents such as aliphatic and aromatic hydrocarbons. |
| Vapor Pressure | 0.00000154 [mmHg] |
| Stability/Shelf Life | /2-(hydroxymethyl)-2-nitro-1,3-propanediol/ is stable only under acidic conditions of pH 5.0 and below, and is unstable and decomposes to formaldehyde under alkaline conditions and at temperatures above 49 °C. |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitrogen oxide/. |
| Chemical Classes | Other Uses -> Biocides/Disinfectants |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 151.12 g/mol |
|---|---|
| XLogP3 | -2.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 151.04807239 g/mol |
| Monoisotopic Mass | 151.04807239 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 107 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
