122-00-9
Product Name:
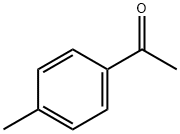
4'-Methylacetophenone
Formula:
C9H10O
Synonyms:
4′-Methylacetophenone;Methyl p-tolyl ketone;Methyl p-tolyl ketone
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Clear, light yellow liquid; mp = 22-24 deg C; [Sigma-Aldrich MSDS] |
|---|---|
| Boiling Point | 222.00 to 226.00 °C. @ 756.00 mm Hg |
| Melting Point | 28 °C |
| Solubility | 0.372 mg/mL at 15 °C |
| Density | 0.999-1.010 |
| Vapor Pressure | 0.22 [mmHg] |
| LogP | 2.10 |
| Refractive Index | 1.530-1.536 |
| Kovats Retention Index | 1157 1157 1161 1162.7 1163.6 1178.2 1181.7 1175.5 1160 1166 1163 1132 1151 1154 1180 1158 1166 1160 1166 1158 1164 1156 1158 1166 1150 1152 |
| Chemical Classes | Other Classes -> Aromatic Ketones |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 134.17 g/mol |
|---|---|
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 134.073164938 g/mol |
| Monoisotopic Mass | 134.073164938 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 121 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
4'-Methylacetophenone is an aromatic ketone,that is found in Citrus medica, Diplolophium africanum, and other organisms with data available.
RELATED SUPPLIERS
All suppliers(7)
