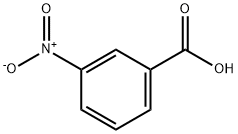
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | M-nitrobenzoic acid appears as off white to yellowish-white crystals. Bitter taste. Melts in hot water. (NTP, 1992) |
|---|---|
| Color/Form | Monoclinic leaflets or prisms |
| Taste | Bitter taste |
| Melting Point | 284 to 288 °F (NTP, 1992) |
| Solubility | less than 0.1 mg/mL at 64 °F (NTP, 1992) |
| Density | 1.494 at 68 °F (NTP, 1992) - Denser than water; will sink |
| Vapor Pressure | 0.0000371 [mmHg] |
| LogP | log Kow = 1.83 |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitrogen oxides/. |
| Heat of Combustion | 3053 kJ/mol |
| Dissociation Constants | K at 25 °C = 3.48X10-4 /pKa = 3.46/ |
| Other Experimental Properties | Heat of fusion = 29.33 kJ/mol; heat capacity = 1.035 J/g-K |
| Chemical Classes | Nitrogen Compounds -> Nitrobenzoic Acids |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 167.12 g/mol |
|---|---|
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 167.02185764 g/mol |
| Monoisotopic Mass | 167.02185764 g/mol |
| Topological Polar Surface Area | 83.1 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 198 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
M-nitrobenzoic acid appears as off white to yellowish-white crystals. Bitter taste. Melts in hot water. (NTP, 1992)
RELATED SUPPLIERS
Orchem Products
Mumbai
product:Powder Technical Grade Meta Nitrobenzoic Acid, For Pharma, Purity: 97%