CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Colorless crystals |
|---|---|
| Melting Point | 77.5-80 °C |
| Solubility | In acetone, hexane 33-50, acetone, ethyl acetate 400-500, n-chlorobutane, chloroform, toluene, N-methyl-2-pyrrolidone >500, dichloromethane >600, dimethylformamide 300-400, ethylene glycol <5, isopropanol, methanol 50-100 (all in g/L at 23 °C) |
| Density | 1.16 |
| Vapor Pressure | 3.4X10-3 mPa /2.55X10-8 mm Hg/ at 25 °C |
| LogP | log Kow = 5.51 at 20 °C |
| Stability/Shelf Life | DT50 of aqueous solution exposed to sunlight is 15 days (pH7, 25 °C) |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitrogen oxides/. |
| Dissociation Constants | pKa = 2.44 |
| Kovats Retention Index | 2499 2511 2526.8 2501.6 |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H332:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 306.4 g/mol |
|---|---|
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 306.173213330 g/mol |
| Monoisotopic Mass | 306.173213330 g/mol |
| Topological Polar Surface Area | 35 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 357 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
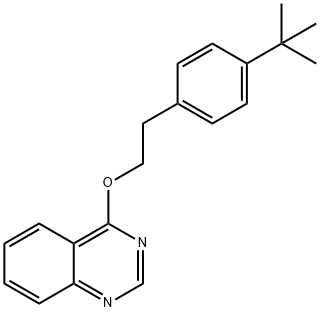
Fenazaquin is a member of quinazolines. It has a role as an acaricide and a mitochondrial NADH:ubiquinone reductase inhibitor.