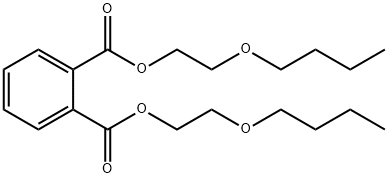
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless liquid; [Hawley] |
|---|---|
| Color/Form | Colorless liquid |
| Boiling Point | 270 °C |
| Melting Point | FP: -55 °C |
| Flash Point | 407 °F; 208 °C (CLOSED CUP) |
| Solubility | Phthalate esters are soluble to various extents in many common organic solvents and oils, but have a relatively low solubility in water. /Phthalate esters/ |
| Density | 1.06 at 20 °C |
| Vapor Pressure | 0.00217 [mmHg] |
| Stability/Shelf Life | Fast to light |
| Decomposition | When heated to decomposition it emits acrid smoke and irritating fumes. |
| Viscosity | 42 mPa sec at 20 °C. |
| Refractive Index | Refractive index = 1.486 at 20 °C/D. |
| Kovats Retention Index | 2484 2485 2485 2485 2486 2486 2488 |
| Other Experimental Properties | Wt/gal = 8.86 lb |
| Chemical Classes | Plastics & Rubber -> Phthalate Esters |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H320:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. |
COMPUTED DESCRIPTORS
| Molecular Weight | 366.4 g/mol |
|---|---|
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 16 |
| Exact Mass | 366.20423867 g/mol |
| Monoisotopic Mass | 366.20423867 g/mol |
| Topological Polar Surface Area | 71.1 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 349 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Bis(2-butoxyethyl)phthalate is a phthalate ester obtained by the formal condensation of both the carboxy groups of phthalic acid with two molecules of 2-butoxyethanol. It has a role as a xenobiotic. It is a diester and a phthalate ester. It is functionally related to a 2-butoxyethanol.