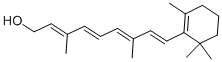
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Vitamin a appears as yellow crystals or orange solid. Practically water insoluble. (NTP, 1992) |
|---|---|
| Color/Form | Solvated crystals from polar solvents, such as methanol or ethyl formate |
| Boiling Point | 279 to 280 °F at 1e-06 mmHg (NTP, 1992) |
| Melting Point | 144 to 147 °F (NTP, 1992) |
| Solubility | Practically insoluble (NTP, 1992) |
| Vapor Pressure | 0.00000008 [mmHg] |
| LogP | 5.68 |
| Henry's Law Constant | Henry's Law constant: 1.2X10-4 atm-cu m/mol @ 25 °C /Estimated/ |
| Stability/Shelf Life | Free alcohol is sensitive to air-oxidation, but oil solutions of it are quite stable; UV light inactivates Vitamin A. |
| Refractive Index | Index of refraction: 1.6410 at 20 °C/D (calculated form refractive indexes of 20-70% solutions in mineral oil); max absorption: 324-325 nm (E=1,835, 1%, 1 cm) |
| Kovats Retention Index | 2453 2453 |
| Other Experimental Properties | Distills at 120-125 °C at 5x10-3 mm pressure. Solution exhibits a yellow-green fluorescence when irradiated with extreme UV light. |
| Chemical Classes | Biological Agents -> Vitamins and Derivatives |
COMPUTED DESCRIPTORS
| Molecular Weight | 286.5 g/mol |
|---|---|
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 5 |
| Exact Mass | 286.229665576 g/mol |
| Monoisotopic Mass | 286.229665576 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 496 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Vitamin a appears as yellow crystals or orange solid. Practically water insoluble. (NTP, 1992)