108-22-5
Product Name:
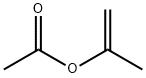
Isopropenyl acetate
Formula:
C5H8O2
Synonyms:
1-Methylvinyl acetate;Acetone enol acetate;IPA;Isopropenyl acetate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Isopropenyl acetate appears as a clear colorless liquid. Less dense than water. Vapors heavier than air. Used to make other chemicals. |
|---|---|
| Color/Form | WATER WHITE LIQUID |
| Boiling Point | 97 °C |
| Melting Point | -92.9 °C |
| Flash Point | 60 °F (NFPA, 2010) |
| Solubility | Soluble in ethanol and acetone, very soluble in ethyl ether. |
| Density | 0.9090 g/cu cm at 20 °C |
| Vapor Pressure | 45.2 [mmHg] |
| Autoignition Temperature | 808 °F (431 °C) |
| Decomposition | When heated to decomposition it emits acrid smoke and irritating fumes. |
| Refractive Index | INDEX OF REFRACTION: 1.4001 AT 20 °C/D |
| Kovats Retention Index | 641 644 673 |
| Other Experimental Properties | CONVERSION FACTORS: 1 MG/L= 245 PPM; 1 PPM= 4.1 MG/CU M |
| Chemical Classes | Other Classes -> Esters, Other |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
COMPUTED DESCRIPTORS
| Molecular Weight | 100.12 g/mol |
|---|---|
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 100.052429494 g/mol |
| Monoisotopic Mass | 100.052429494 g/mol |
| Topological Polar Surface Area | 26.3 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 94.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Isopropenyl acetate appears as a clear colorless liquid. Less dense than water. Vapors heavier than air. Used to make other chemicals.
RELATED SUPPLIERS
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
Gujarat
product:Isopropenyl Acetate (108-22-5)
