1070505-53-1
Product Name:
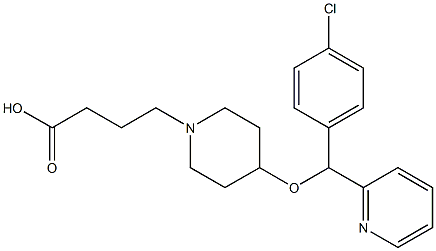
4-[(4-Chlorophenyl)-2-pyridinylmethoxy]-1-piperidinebutanoic acid
Formula:
C21H25ClN2O3
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Solubility | 5.03e-02 g/L |
COMPUTED DESCRIPTORS
| Molecular Weight | 388.9 g/mol |
|---|---|
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 388.1553704 g/mol |
| Monoisotopic Mass | 388.1553704 g/mol |
| Topological Polar Surface Area | 62.7 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 449 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Bepotastine is a non-sedating, selective antagonist of the histamine 1 (H1) receptor. Bepotastine was approved in Japan for use in the treatment of allergic rhinitis and uriticaria/puritus in July 2000 and January 2002, respectively, and is marketed by Tanabe Seiyaku Co., Ltd. under the brand name Talion. It is available in oral and opthalmic dosage forms in Japan. The opthalmic solution is FDA approved since Sept 8, 2009 and is under the brand name Bepreve.