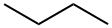CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Butane is a colorless gas with a faint petroleum-like odor. For transportation it may be stenched. It is shipped as a liquefied gas under its vapor pressure. Contact with the liquid can cause frostbite. It is easily ignited. Its vapors are heavier than air. Any leak can be either liquid or vapor. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. It is used as a fuel, an aerosol propellant, in cigarette lighters, and to make other chemicals. |
|---|---|
| Color/Form | Colorless gas [Note: Shipped as a liquefied compressed gas. A liquid below 31 degrees F] |
| Odor | Faint, disagreeable odor |
| Boiling Point | 31.1 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -217.1 °F (NTP, 1992) |
| Flash Point | -76 °F (NTP, 1992) |
| Solubility | 61 mg/L at 68 °F (NTP, 1992) |
| Density | 0.6 at 32 °F (USCG, 1999) - Less dense than water; will float |
| Vapor Density | 2.046 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 760 mmHg at 31.1 °F ; 1823 mmHg at 77 °F (NTP, 1992) |
| LogP | log Kow = 2.89 |
| Autoignition Temperature | 550 °F (USCG, 1999) |
| Decomposition | When heated to decomposition it emits acrid smoke and fumes. |
| Viscosity | 7.5 at 300 K; 9.9 at 400 K; 12.2 at 500 K; 14.5 at 600 K (all in uPa.s) (gas) |
| Corrosivity | Has no corrosive action on metals |
| Heat of Combustion | -19,512 BTU/lb= -10,840 cal/g= -453.85x10+5 J/kg |
| Heat of Vaporization | 22.39 kJ/mol at normal BP |
| Surface Tension | 14.7 dynes/cm at 0 °C |
| Ionization Potential | 10.63 eV |
| Odor Threshold | Odor Threshold Low: 1.2 [ppm] Odor Threshold High: 6.5 [ppm] Odor threshold from ACGIH |
| Refractive Index | Index of refraction: 1.3326 at 20 °C/D |
| Kovats Retention Index | 400 |
| Other Experimental Properties | Condensing pressure: approx 30 lb at 32.5 °C; specific volume: 6.4 cu ft/lb at 21.1 °C; does not react with moisture |
| Chemical Classes | Solvents -> Aliphatics, Saturated (<C12) |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Gas Cylinder Compressed Gases GHS04 |
| GHS Hazard Statements |
H220:Flammable gases H280:Gases under pressure |
| Precautionary Statement Codes |
P410+P403:Protect from sunlight. Store in a well-ventilated place. |
COMPUTED DESCRIPTORS
| Molecular Weight | 58.12 g/mol |
|---|---|
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 1 |
| Exact Mass | 58.078250319 g/mol |
| Monoisotopic Mass | 58.078250319 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Butane is a colorless gas with a faint petroleum-like odor. For transportation it may be stenched. It is shipped as a liquefied gas under its vapor pressure. Contact with the liquid can cause frostbite. It is easily ignited. Its vapors are heavier than air. Any leak can be either liquid or vapor. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. It is used as a fuel, an aerosol propellant, in cigarette lighters, and to make other chemicals.