106-88-7
Product Name:
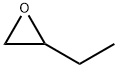
1,2-EPOXYBUTANE
Formula:
C4H8O
Synonyms:
1,2-Epoxybutane;1-Butene oxide;Ethyloxirane;Ethyloxirane, 1,2-Butylene oxide;α-Butylene oxide
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | 1,2-butylene oxide appears as a clear colorless volatile liquid with an ethereal odor. Flash point near 0 °F. Density about 6.9 lb / gal. Soluble in water. Boiling point near 140 °F. Flammable over a wide range of vapor-air concentrations. May polymerize with the evolution of heat and possible rupture of container if contaminated. Vapors irritate eyes, skin and respiratory system. Prolonged contact with skin may cause in delayed burns. Vapors are heavier than air. Used as an intermediate to make various polymers. Chemicals that polymerize are often stabilized by refrigeration. |
|---|---|
| Color/Form | Colorless liquid |
| Odor | Disagreeable odor |
| Boiling Point | 145 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -76 °F (NTP, 1992) |
| Flash Point | 10 °F (NTP, 1992) |
| Solubility | greater than or equal to 100 mg/mL at 63 °F (NTP, 1992) |
| Density | 0.826 at 77 °F (USCG, 1999) - Less dense than water; will float |
| Vapor Density | 2.49 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 160 mmHg at 55 °F ; 215 mmHg at 70.0 °F (NTP, 1992) |
| LogP | log Kow = 0.68 (OECD Method 107) |
| Autoignition Temperature | 959 °F (USCG, 1999) |
| Decomposition | When heated to decomposition it emits acrid smoke and fumes. |
| Viscosity | 0.40 mPa.s at 25 °C |
| Heat of Combustion | -15,200 btu/lb (-8,470 cal/g or -3.54X10+7 J/kg) |
| Heat of Vaporization | 30.3 kJ/mol at 63.4 °C |
| pH | Approximately 7 at 50 g/L at 20 °C |
| Surface Tension | 23.5 mN/m at 25 °C |
| Polymerization | Hazardous polymerization may occur. Usually contains inhibitors to prevent polymerization. Uninhibited monomer vapor may form polymer in vents and other confined spaces. |
| Odor Threshold | Odor Threshold Low: 0.07 [mmHg] Odor Threshold High: 0.71 [mmHg] [ICSC] Odor threshold low (absolute perception limit), and high (recognition) from CHEMINFO |
| Refractive Index | Index of refraction: 1.3851 at 20 °C |
| Kovats Retention Index | 587 587 588 587 587.4 587.8 551 552 557 579 582 582 600 600 |
| Other Experimental Properties | Liquid is lighter than water, vapor is heavier than air |
| Chemical Classes | Plastics & Rubber -> Epoxides |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 72.11 g/mol |
|---|---|
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 72.057514874 g/mol |
| Monoisotopic Mass | 72.057514874 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 34.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
1,2-butylene oxide appears as a clear colorless volatile liquid with an ethereal odor. Flash point near 0 °F. Density about 6.9 lb / gal. Soluble in water. Boiling point near 140 °F. Flammable over a wide range of vapor-air concentrations. May polymerize with the evolution of heat and possible rupture of container if contaminated. Vapors irritate eyes, skin and respiratory system. Prolonged contact with skin may cause in delayed burns. Vapors are heavier than air. Used as an intermediate to make various polymers. Chemicals that polymerize are often stabilized by refrigeration.
RELATED SUPPLIERS
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
Gujarat
product:1,2-Epoxybutane (106-88-7) (C4H8O), 96-98%, 165 kg drum
