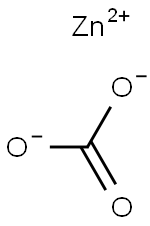
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Zinc carbonate appears as a white crystalline solid or powder that is insoluble in water. The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Used in pharmaceuticals, to make other zinc compounds, as a feed additive. |
|---|---|
| Color/Form | WHITE RHOMBIC CRYSTALS |
| Odor | Odorless |
| Melting Point | Loses CO2 at 572 °F = 300 °F = 573.2 °F (USCG, 1999) |
| Solubility | 0.001 G/100 G OF WATER AT 15 °C; SOL IN DIL ACIDS, ALKALIES & SOLN OF AMMONIUM SALTS. |
| Density | 4.398 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | When heated to decomp ... emits toxic fumes of /carbon monoxide and zinc/. |
| Refractive Index | INDEX OF REFRACTION: 1.818, 1.618 |
| Other Experimental Properties | EVOLVES CARBON DIOXIDE AT 300 °C |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 125.4 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 123.913886 g/mol |
| Monoisotopic Mass | 123.913886 g/mol |
| Topological Polar Surface Area | 63.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Zinc carbonate appears as a white crystalline solid or powder that is insoluble in water. The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Used in pharmaceuticals, to make other zinc compounds, as a feed additive.