102691-36-1
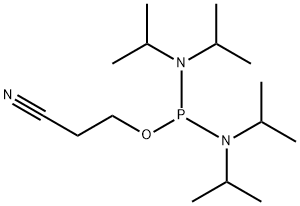
Product Name:
2-Cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite
Formula:
C15H32N3OP
Synonyms:
Bis(diisopropylamino)(2-cyanoethoxy)phosphine
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H317:Sensitisation, Skin H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
COMPUTED DESCRIPTORS
| Molecular Weight | 301.41 g/mol |
|---|---|
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 9 |
| Exact Mass | 301.22829965 g/mol |
| Monoisotopic Mass | 301.22829965 g/mol |
| Topological Polar Surface Area | 39.5 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 274 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
Description
2-Cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite is an important organic reagent widely used in the preparation of modified and unmodified nucleoside phosphoramidites. Nucleoside phosphoramidites are ideal reagents for polymer-supported synthesis of oligonucleotides. The diisopropylamino leaving group is readily cleaved upon contact with the azole catalyst, resulting in an efficient coupling reaction with the free hydroxyl group on the protected nucleoside.