102-27-2
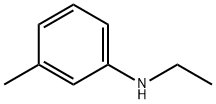
Product Name:
N-Ethyl-3-methylaniline
Formula:
C9H13N
Synonyms:
N-Ethyl-3-aminotoluene;N-Ethyl-3-methylaniline;3-(Ethylamino)toluene
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | N-ethyl-m-toluidine appears as a light amber liquid. Less dense than water and insoluble in water. Vapors heavier than air. Produces toxic oxides of nitrogen during combustion. Used to make other chemicals. |
|---|---|
| Color/Form | Light-amber liquid |
| Boiling Point | 221 °C |
| Solubility | Sol in ethanol and ethyl ether |
| Vapor Pressure | 0.13 [mmHg] |
| Refractive Index | Index of refraction: 1.5451 @ 20 °C/D; |
| Kovats Retention Index | 1200 |
| Chemical Classes | Nitrogen Compounds -> Amines, Aromatic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 135.21 g/mol |
|---|---|
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 135.104799419 g/mol |
| Monoisotopic Mass | 135.104799419 g/mol |
| Topological Polar Surface Area | 12 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 90.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
N-ethyl-m-toluidine appears as a light amber liquid. Less dense than water and insoluble in water. Vapors heavier than air. Produces toxic oxides of nitrogen during combustion. Used to make other chemicals.
