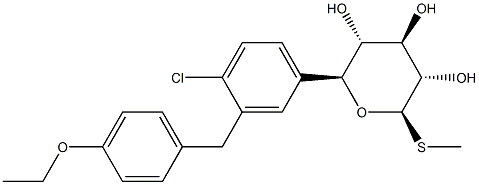
COMPUTED DESCRIPTORS
| Molecular Weight | 424.9 g/mol |
|---|---|
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 424.1111228 g/mol |
| Monoisotopic Mass | 424.1111228 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 476 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sotagliflozin is a dual inhibitor of SGLT1 and SGLT2, the first of its kind, which is approved for use in the EU, in combination with insulin, to improve glycemic control in patients with type 1 diabetes mellitus (T1DM) and a BMI ≥27 kg/m2. Its potency in inhibiting SGLT2 is similar to that of other SGLT2 inhibitors, such as [canagliflozin] and [dapagliflozin], but its potency in inhibiting SGLT1 is >10-fold higher than its predecessors. The added inhibition of intestinal SGLT1 delays glucose absorption in the distal small intestine and colon, thereby reducing post-prandial glucose levels. Sotagliflozin was approved in the EU under the brand name "Zynquista" on April 26, 2019. A similar approval has also been sought in the US, but the FDA has since published a proposal to refuse the approval on the grounds that the data submitted did not show that it was safe under the proposed conditions of use.