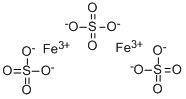CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ferric sulfate appears as a yellow crystalline solid or a grayish-white powder. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. It is used for water purification, and as a soil conditioner. |
|---|---|
| Color/Form | Grayish-white powder, or rhombic or rhombohedral crystals |
| Melting Point | 480 °C (decomp) |
| Solubility | Slowly sol in water; rapidly sol in the presence of a trace of ferrous sulfate; sparingly sol in alc; practically insol in acetone, ethyl acetate. |
| Density | 3.1 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Stability/Shelf Life | Hydrolyzed slowly in aqueous solution. |
| Decomposition | When heated to decomposition it emits toxic fumes /of iron and sulfur oxides./ |
| Corrosivity | Corrosive to copper, copper alloys, mild steel, and galvanized steel. |
| Refractive Index | Index of refraction: 1.814 |
| Other Experimental Properties | MW 562.00; rhombic crystals; deliquescent; index of refraction 1.552, 1.553; density 2.1; MP 175 °C (loses 7H2O); solubility 440 g/100 ml in cold water; decomp in hot water; sol in abs alc. /Iron(III) sulfate, enneahydrate/ |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 399.9 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 0 |
| Exact Mass | 399.725060 g/mol |
| Monoisotopic Mass | 399.725060 g/mol |
| Topological Polar Surface Area | 266 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 5 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ferric sulfate appears as a yellow crystalline solid or a grayish-white powder. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. It is used for water purification, and as a soil conditioner.