10026-03-6
Product Name:
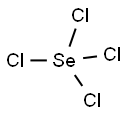
Selenium tetrachloride
Formula:
Cl4Se
Synonyms:
Selenium (Ⅳ) chloride
Inquiry
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 220.8 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 221.78898 g/mol |
| Monoisotopic Mass | 219.79193 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 19.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Selenium tetrachloride is a chloride of selenium. Selenium is a nonmetal element with the atomic number 34 and the chemical symbol Se. Selenium rarely occurs in its elemental state in nature and is usually found in sulfide ores such as pyrite, partially replacing the sulfur in the ore matrix. It may also be found in silver, copper, lead, and nickel minerals. Though selenium salts are toxic in large amounts, trace amounts of the element are necessary for cellular function in most animals, forming the active center of the enzymes glutathione peroxidase, thioredoxin reductase, and three known deiodinase enzymes. (L620)
