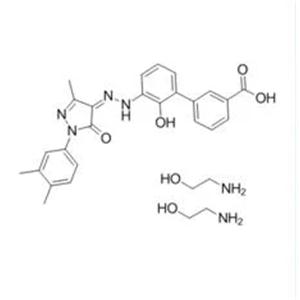
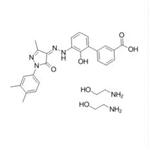




Unii-4u07f515LG; Eltrombopag Olamine; CAS 496775-62-3
| Price | USD10.00 |
| Packge | 1kg |
- Min. Order:20kg
- Supply Ability:500tons
- Time:2022-06-28
Product Details
- Product NameEltrombopag Olamine
- CAS No.496775-62-3
- EINECS No.629-876-8
- MFC27H29N5O5
- MW503.56
- AppearancePowder
- storage temp. under inert gas (nitrogen or Argon) at 2–8 °C
Basic Info.
Colour
White
Samples
Available
Purity
99%
Certificates
ISO9001/SGS
Package
1kg/5kg/10kg/25kg
Stocks
in Stock
Lead Time
5-7 Working Days
Shelf Life
2-3 Years
Site Audit
Available
Transport
Air/Express/Sea
MOQ
10g
Transport Package
Drum/Bottle/Bags
Product Description
Unii-4U07F515lg;Eltrombopag olamine;CAS 496775-62-3 

| Unii-4U07F515lg Chemical Properties |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| Safety Information |
| MSDS Information |
| Unii-4U07F515lg Usage And Synthesis |
| Uses | Treatment of chemotherapy-induced thrombocytopenia and treatment of immune thrombocytopenic purpura. |
| Clinical Use | Eltrombopag olamine, a thrombopoietin receptor (TpoR) agonist, was approved in late 2008 for the once-daily, oral short-term and long-term treatment of adult patients with previously treated chronic idiopathic thrombocytopenic purpura (ITP). It is the first small-molecule TpoR agonist and was launched in the U.S. for this indication in 2009 by GlaxoSmithKline (GSK). Because eltrombopag is a small molecule, the drug is administered orally and has a reduced potential for causing an immune system reaction versus alternative protein-based therapies. In 2010, eltrombopag was approved in Europe for the long-term treatment of adult patients with previously treated chronic ITP. |
| Chemical Synthesis | The synthesis began with the nitration of 2-bro mophenol (39) with sodi um nitrate and sulfuric acid in water at 10°C to give 2-bromo-6-nitrophenol (40) in 25% yield, which was methylated using met hyl iodide and potassium carbonate in refluxing aceto ne providing 2-bromo- 6-nitroanisole (41) in 76% yield (the Scheme).40 Suzuki coupling of compound 41 with 3-carboxyphenyl boronic acid with Pd(PPh3)4 and 2 M sodium carbonate in refluxing dio xane gave 20-methoxy- 30-nitrobiphenyl-3-carboxylic acid (42) in 47% yield as a tan powder. Demethylation using 48% HBr (aq) in refluxing acetic acid resulted in a 79% yield of 20-hydroxy-30-nitrobiphenyl-3-carboxylic acid (43). The nitro group of compound 43 was reduced via catalytic hydrogenation at 50 psi at room temperature over Pd/C in mixed ethanol/3 M aq NaOH solution to give 30-amino-20-hydroxybiphenyl- 3-carboxylic acid (44) in quantitative yield. The intermediate 1-(3,4-dimethylphenyl)-3-methyl-2,5-dihydro-1Hpyrazol- 5-one (47) was prepared by condensing of 3,4-dimethylphenyl- hydrazine 45 with ethyl acetoacetate 46 with sodium acetate in refluxing acetic acid in 76% yield. Treatment of (44) with sod ium nitrite in 1 M HCl at 5°C, followed by condensation with 1-(3,4-dimethylphenyl)-3-methyl-2,5-dihydro-1H-pyrazol-5-one (47) at a constant pH of 7-8 via the addition of sodium bicarbonate and ethanol afforded eltrombopag in 32% yield. Finally, eltrombopag was treated with hydroxyl ethyla mine to give eltrombopag olamine (VIII). |

Company Profile Introduction
New material technology research and development、Technology popularization、
Technical consulting、Technology transfer、Medical technology r&d
Recommended supplier
-
VIP1年
- Granules India Limited
- Eltrombopag Olamine 496775-62-3 99%
- Inquiry
- 2024-03-22
-
VIP1年
- Beta Drugs
- Eltrombopag Olamine 496775-62-3 98%
- Inquiry
- 2024-02-26
-
VIP1年
- Biophore India Pharmaceuticals Pvt Ltd
- Eltrombopag Olamine 98%
- Inquiry
- 2024-02-21
-
VIP1年
- Zydus Lifesciences Ltd.
- 496775-62-3 98%
- Inquiry
- 2024-01-27
-
VIP1年
- Avra Laboratories Pvt Ltd
- 496775-62-3 Eltrombopag Olamine 99%
- Inquiry
- 2024-01-09
-
VIP1年
- Metrochem API Private Limited
- Eltrombopag Olamine 98%
- Inquiry
- 2024-01-03
- Since:2022-03-18
- Address: Room 601, Unit 1, Building 1, 188 Xinhuali, North Xinhua Road, Xiangdu District, Xingtai city, Hebei
INQUIRY
