

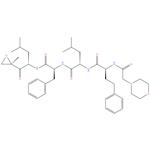
Carfilzomib 98%
| Price | Get Latest Price | ||||
| Packge | 1MT | 1kg | 5kg | 10kg | 25kg |
- Min. Order:1MT
- Time:2024-03-06
Product Details
- Product NameCarfilzomib
- CAS No.868540-17-4
- EINECS No.692-054-2
- MFC40H57N5O7
- MW719.92
- AppearancesolidWhite
- density 1.161
- Boiling point 975.6±65.0 °C(Predicted)
- storage temp. -20°
- Melting point 204 - 208°C
Company Profile Introduction
Mylan N.V. and Pfizer Inc concluded a global transaction combining Mylan N.V. and Upjohn (Pfizer’s off-patent branded and generic established medicines business) to create a new pharmaceutical company called Viatris Inc in 2020. Following the global transaction, Mylan Laboratories Limited and Mylan Pharmaceuticals Private Limited, the legal entities operating in India, became subsidiaries of Viatris Inc.
We are a global healthcare company uniquely positioned to bridge the traditional divide between generics and brands, combining the best of both, to more holistically address healthcare needs globally. With a mission to empower people worldwide to live healthier at every stage of life, we provide access at scale.
At Viatris, we see healthcare not as it is, but as it should be. We act courageously and are uniquely positioned to be a source of stability in a world of evolving healthcare needs.
Supplier other products
Recommended supplier
-
VIP1年
- Vijaya Pharma And Life Science
- Carfilzomib 868540-17-4 98%
- Inquiry
- 2024-03-13
-
VIP1年
- Apicore Pharmaceuticals Pvt Ltd
- Carfilzomib 868540-17-4 98%
- Inquiry
- 2024-03-07
-
VIP1年
- Beta Drugs
- 868540-17-4 98%
- Inquiry
- 2024-02-26
-
VIP1年
- Biophore India Pharmaceuticals Pvt Ltd
- 868540-17-4 Carfilzomib 98%
- Inquiry
- 2024-02-21
-
VIP1年
- Sakar Healthcare
- Carfilzomib 868540-17-4 98%
- Inquiry
- 2024-02-05
-
VIP1年
- Basil Drugs AND Pharmaceuticals Pvt Ltd
- Carfilzomib 98%
- Inquiry
- 2024-01-23
-
VIP1年
- Avra Laboratories Pvt Ltd
- 868540-17-4 99%
- Inquiry
- 2024-01-09
- Since:2021-03-01
- Address: Plot No 564/A/22, Road No 92, Jubilee Hills, Hyderabad, Telangana-500034, India.



