

Mezlocillin
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:10000KGS
- Time:2020-01-01
Product Details
- Product NameMezlocillin
- CAS No.51481-65-3
- EINECS No.257-233-8
- MFC21H25N5O8S2
- MW539.58
- density 1.63±0.1 g/cm3(Predicted)
- storage temp. 2-8°C
hanne 774
▼
▲
Product Name:
Synonyms:
(2s-(2-alpha,5-alpha,6-beta)(s*))-sodiumsal;4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylicacid,3,3-dimethyl-6-(((((3-(met;3,3-dimethyl-7-[[2-[(3-methylsulfonyl-2-oxo-imidazolidin-1-yl)carbonylamino]-2-phenyl-acetyl]amino]-6-oxo-2-thia-5-azabicyclo[3.2.0]heptane-4-carboxylic acid;MEZLOCILLIN;Melocillin sodium;antibioticbay-f1353;Meloxacam acid;Mezlocillin acid
CAS:
MF:
C21H25N5O8S2
MW:
539.58
EINECS:
257-233-8
Product Categories:
Mol File:
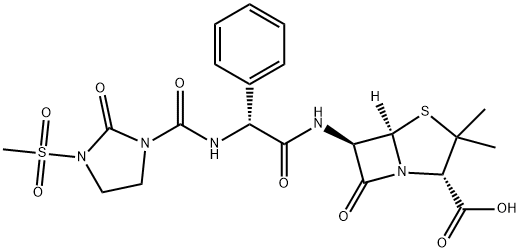
▼
▲
Mezlocillin Chemical Properties
▼
▲
density
1.63±0.1 g/cm3(Predicted)
pka
pKa 2.7 (Uncertain)
CAS DataBase Reference
▼
▲
Safety Information
▼
▲
▼
▲
Mezlocillin Usage And Synthesis
▼
▲
Uses
Antibacterial.
Definition
ChEBI: A penicillin in which the substituent at position 6 of the penam ring is a (2R)-2-[3-(methanesulfonyl)-2-oxoimidazolidine-1-carboxamido]-2-phenylacetamido group.
Brand name
Multocillin (Bayer).
Antimicrobial activity
A semisynthetic acylureidopenicillin supplied as the sodium salt for parenteral administration.
Ampicillin-susceptible strains of H. influenzae and Neisseria spp. are very susceptible, but β-lactamase-producing organisms are usually resistant. It is less active than azlocillin and piperacillin against Ps. aeruginosa and has variable activity against B. fragilis, independent of β-lactamase production. It exhibits typical β-lactam synergy with aminoglycosides against Ps. aeruginosa and enterobacteria.
It attains peak concentrations of 250 mg/L after a 2 g intravenous infusion, with a plasma half-life of 55 min. Protein binding is 20–30%. It distributes into multiple tissues and human body fluids at therapeutically useful concentrations. Up to 60% of the dose is recoverable unchanged from the urine, with up to 2.5% excreted in the bile.
Toxicity and side effects are similar to those associated with carboxypenicillins. Its clinical use is for serious infections with susceptible organisms, including lower respiratory tract, intra-abdominal, urinary tract and gynecological infections. Commercial availability is quite limited.
Ampicillin-susceptible strains of H. influenzae and Neisseria spp. are very susceptible, but β-lactamase-producing organisms are usually resistant. It is less active than azlocillin and piperacillin against Ps. aeruginosa and has variable activity against B. fragilis, independent of β-lactamase production. It exhibits typical β-lactam synergy with aminoglycosides against Ps. aeruginosa and enterobacteria.
It attains peak concentrations of 250 mg/L after a 2 g intravenous infusion, with a plasma half-life of 55 min. Protein binding is 20–30%. It distributes into multiple tissues and human body fluids at therapeutically useful concentrations. Up to 60% of the dose is recoverable unchanged from the urine, with up to 2.5% excreted in the bile.
Toxicity and side effects are similar to those associated with carboxypenicillins. Its clinical use is for serious infections with susceptible organisms, including lower respiratory tract, intra-abdominal, urinary tract and gynecological infections. Commercial availability is quite limited.
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY
