

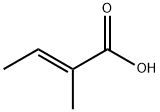
Tiglic acid
| Price | USD1.00 | USD1.00 |
| Packge | 1kg | 1kg |
- Min. Order:1 mg
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameTiglic acid
- CAS No.80-59-1
- EINECS No.201-295-0
- MFC5H8O2
- MW100.12
- InChIKeyUIERETOOQGIECD-ONEGZZNKSA-N
- AppearanceCrystalline Powder and ChunksWhite to beige
- Boiling point 95-96 °C12 mm Hg(lit.)
- density 0.969 g/mL at 25 °C(lit.)
- Water Solubility SOLUBLE IN HOT WATER
- Melting point 61-64 °C(lit.)
- storage temp. 2-8°C
| Tiglic acid Basic information |
| Product Name: | Tiglic acid |
| Synonyms: | (E)-2-METHYL-2-BUTENOIC ACID;E-2,3-DIMETHYLACRYLIC ACID;FEMA 3599;2,3-DIMETHYLACRYLIC ACID;2-METHYL-TRANS-2-BUTENOIC ACID;2-METHYL-2-BUTENOIC ACID;2-METHYLBUT-2-ENOIC ACID;2-METHYLCROTONIC ACID |
| CAS: | 80-59-1 |
| MF: | C5H8O2 |
| MW: | 100.12 |
| EINECS: | 201-295-0 |
| Product Categories: | Aromatic Cinnamic Acids, Esters and Derivatives |
| Mol File: | 80-59-1.mol |
 |
|
| Tiglic acid Chemical Properties |
| Melting point | 61-64 °C(lit.) |
| Boiling point | 95-96 °C12 mm Hg(lit.) |
| density | 0.969 g/mL at 25 °C(lit.) |
| FEMA | 3599 | 2-METHYL-TRANS-2-BUTENOIC ACID |
| refractive index | nD81 1.4342 |
| Fp | 101°C |
| storage temp. | Refrigerator (+4°C) |
| pka | pK (25°) 5.02 |
| form | Crystalline Powder and Chunks |
| color | White to beige |
| Water Solubility | SOLUBLE IN HOT WATER |
| Merck | 14,9433 |
| BRN | 1236500 |
| CAS DataBase Reference | 80-59-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Butenoic acid, 2-methyl-, (E)-(80-59-1) |
| EPA Substance Registry System | 2-Butenoic acid, 2-methyl-, (2E)-(80-59-1) |
| Safety Information |
| Hazard Codes | C,Xi |
| Risk Statements | 34-36/37/38 |
| Safety Statements | 26-36/37/39-45-24/25-27 |
| RIDADR | UN 3261 8/PG 2 |
| WGK Germany | 2 |
| RTECS | GQ5430000 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 29161980 |
| Hazardous Substances Data | 80-59-1(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| Tiglic acid | English |
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| Tiglic acid Usage And Synthesis |
| Description | Tiglic acid (also (E)-2-Methylbut-2-enoic acid) is an organic acid. It occurs in croton oil, Roman chamomile, and geranium. It can be prepared by dehydration of MEK cyanohydrin, followed by the hydrolysis of the nitrile function. It is either in the form of a thick syrup or colorless crystals. It is with a sweet, warm, spicy odor. It is used to synthesize esters for perfume manufacturing industry and as a raw material to produce acids and esters, which are applied as flavoring agent for food and beverage industry. Tiglic acid is also a useful starting material in the synthesis of pharmaceutical intermediates. |
| References | [1] H. Panda (2010) Perfumes and Flavours Technology Handbook. [2] Paul T. Anastas (2009) Handbook of Green Chemistry, Green Catalysis, Biocatalysis. [3] https://en.wikipedia.org/wiki/Tiglic_acid |
| Chemical Properties | WHITE TO BEIGE CRYSTALLINE POWDER AND CHUNKS |
| Uses | Tiglic acid is the stable isomer of angelic acid. Tiglic acid was found as glyceride in croton oil, as butyl ester in the oil of the Roman camomile, and as geranyl tiglate in oil of geranium. Tiglic a cid is formed during the charcoaling of maple wood. |
| Definition | ChEBI: A 2-methylbut-2-enoic acid having its double bond in trans-configuration. |
| General Description | A white lustrous flaked solid with a fruity or spicy odor. About the same density as water and slightly soluble in water. May burn if heated to high temperatures. May severely irritate skin, eyes, lungs or mucous membranes. |
| Air & Water Reactions | Slightly soluble in water. |
| Reactivity Profile | TIGLIC ACID is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Tiglic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. |
| Health Hazard | TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
| Fire Hazard | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
| Safety Profile | Low toxicity by ingestion and skin contact. A severe skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Purification Methods | Crystallise it from water. It is steam volatile and is soluble in organic solvents. [Beilstein 2 IV 1552.] |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- JSK Chemicals
- 80-59-1 trans-2,3-Dimethylacrylic acid, 98% 99%
- Inquiry
- 2023-12-22
-
VIP1年
- Aamirav Ingredients And Specialties Pvt Ltd
- Tiglic acid citronellyl ester 24717-85-9 98%
- Inquiry
- 2024-02-24
-
VIP1年
- Arpan Aromatics
- Tiglic acid citronellyl ester 98%
- Inquiry
- 2024-02-13
-
VIP1年
- Ramsun Bio Organics
- Tiglic acid geranyl ester 98%
- Inquiry
- 2024-01-11
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
