

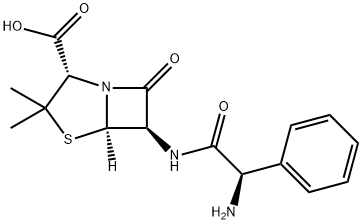
Ampicillin
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1g
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameAmpicillin
- CAS No.69-53-4
- EINECS No.200-709-7
- MFC16H19N3O4S
- MW349.4
- InChIKeyAVKUERGKIZMTKX-NJBDSQKTSA-N
- AppearancesolidWhite to Pale Yellow
- Melting point 198-200 °C (dec.)(lit.)
- storage temp. 2-8°C
- Boiling point 201.8°C (rough estimate)
- density 1.2794 (rough estimate)
- Water Solubility 13.9g/L(25 ºC)
| Ampicillin Basic information |
| Product Name: | Ampicillin |
| Synonyms: | 6-[D-ALPHA-AMINOPHENYLACETAMIDO]PENICILLANIC ACID TRIHYDRATE;ALPHA-AMINOBENZYLPENICILLIN, TRIHYDRATE;D(-)-ALPHA-AMINOBENZYLPENICILLIN;D(-)-ALPHA-AMINOBENZYLPENICILLIN TRIHYDRATE;(aminophenylmethyl)-penicilli;[2s-[2alpha,5alpha,6beta(s)]]-o]-3-dimethyl-7-oxo;4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylicacid,6-[(aminophenylacetyl)amin;6-(d(-)-alpha-aminophenylacetamido)penicillanicacid |
| CAS: | 69-53-4 |
| MF: | C16H19N3O4S |
| MW: | 349.41 |
| EINECS: | 200-709-7 |
| Product Categories: | Chiral Reagents;Intermediates & Fine Chemicals;Isotope Labeled Compounds;Pharmaceuticals;veterinary medicine,soluble powder;Isotope Labelled Compounds;Sulfur & Selenium Compounds;Antibacterial Drugs;Thiazines ,Thiazoles;API |
| Mol File: | 69-53-4.mol |
 |
|
| Ampicillin Chemical Properties |
| Melting point | 198-200 °C (dec.)(lit.) |
| alpha | D23 +287.9° (c = 1 in water) |
| Boiling point | 201.8°C (rough estimate) |
| density | 1.2794 (rough estimate) |
| refractive index | 1.6320 (estimate) |
| storage temp. | 2-8°C |
| solubility | NH4OH 1 M: 50 mg/mL, clear, colorless |
| pka | 2.5 (COOH)(at 25℃) |
| Merck | 13,591 |
| Stability: | Stable, but may be moisture sensitive. Incopmpatible with strong oxidizing agents. |
| CAS DataBase Reference | 69-53-4(CAS DataBase Reference) |
| EPA Substance Registry System | 4-Thia-1-azabicyclo[ 3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-aminophenylacetyl] amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)-(69-53-4) |
| Safety Information |
| Hazard Codes | Xn,T |
| Risk Statements | 36/37/38-42/43-61 |
| Safety Statements | 22-26-36/37-45-53 |
| WGK Germany | 2 |
| RTECS | XH8425000 |
| F | 10-23 |
| HS Code | 29212900 |
| Hazardous Substances Data | 69-53-4(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| Ampicillin | English |
| SigmaAldrich | English |
| Ampicillin Usage And Synthesis |
| Brand Name(s) | Human forms include Omnipen, Principen, Totacillin, Polycillin. Injectable forms include Omnipen-N, Polycillin-N, Totacillin- N. Veterinary forms include Amp-Equine, and Ampicillin trihydrate (Polyflex). |
| Description | Ampicillin is an antibacterial antibiotic from the α-aminobenzyl penicillin group, which differs from penicillin by the presence of an amino group that facilitates penetration through the outer membrane of some gram-negative bacteria. Ampicillin is Semi-synthetic derivative of penicillin that functions as an orally active broad-spectrum antibiotic. Ampicillin acts by interfering directly with the biosynthesis of peptidoglycan, which constitutes the major component of the bacterial cell wall, leading to structural instability and death of bacteria. Ampicillin is a β-lactam antibiotic within the penicillin family. As a member of this family, Ampicillin is susceptible to β-lactamase, which hydrolyzes the β-lactam ring. This broad spectrum antibiotic is effective against gram-positive, gram-negative bacteria and anaerobic bacteria. Ampicillin is widely used in cell culture as a selective agent. As an antibiotic, Ampicillin binds to penicillin binding proteins (PBPs) on a susceptible organism and Inhibits bacterial cell-wall synthesis by inactivating transpeptidases on the inner surface of the bacterial cell membrane. After binding to the PBPs, Ampicilin acts as a structural analogue of acyl-D-alanyl-D-alanine, acylates the transpeptidase enzyme and thereby prevents the cross-linking of the peptidoglycan of the cell wall necessary for the growth of the bacterium. Ampicillin sodium can be used as a selective agent in several types of isolation media. Ampicillin sodium is routinely used to select for cells containing the pcDNA3.1 and pEAK10 resistance plasmids in cell line A904L at an effective concentration of 50 μg/ml. |
| Chemical Properties | Crystalline Solid |
| Uses | β-lactam antibiotics |
| Uses | Labelled Ampicillin. Orally active, semi-synthetic antibiotic; structurally related to penicillin. Antibacterial. |
| Definition | ChEBI: A penicillin in which the substituent at position 6 of the penam ring is a 2-amino-2-phenylacetamido group. |
| Brand name | Amcill (Parke-Davis); Omnipen (Wyeth-Ayerst); Polycillin (Apothecon); Principen (Apothecon). |
| Contact allergens | Ampicillin caused contact dermatitis in a nurse also sensitized to amoxicillin (with tolerance to oral phenoxymethylpenicillin) and in a pharmaceutical factory worker. Systemic drug reactions are common. Crossreactivity is regular with ampicillin and can occur with other penicillins. |
| Safety Profile | Poison by intracerebral route. Moderately toxic by intraperitoneal route. Human systemic effects by ingestion: fever, agranulocytosis, and other blood effects. An experimental teratogen. Mutation data reported. Questionable carcinogen. When heated to decomposition it emits very toxic fumes of NO, and SOx,. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP0年
- Ebenezer Industries
- 69-53-4 98%
- Inquiry
- 2024-03-30
-
VIP1年
- Penam Laboratories Ltd
- Ampicillin 98%
- Inquiry
- 2024-03-29
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
