


Dacomitinib (PF299804)
| Price | USD2.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100kg
- Time:2019-07-06
Product Details
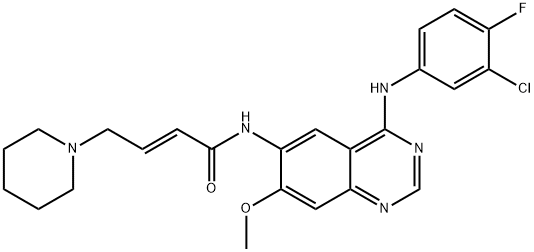
- Product NameDacomitinib (PF299804)
- CAS No.1110813-31-4
- EINECS No.000-000-0
- MFC24H25ClFN5O2
- MW469.94
- AppearanceWhite solid.White to Pale Yellow
- density 1.344
- Boiling point 665.7±55.0 °C(Predicted)
- Melting point 184-187°C
- storage temp. Refrigerator
| Dacomitinib (PF299804) Basic information |
| Product Name: | Dacomitinib (PF299804) |
| Synonyms: | Dacomitinib;PF-00299804-03;PF-00299804;DacoMitinib (PF-00299804);DacoMitinib,PF299804;DacoMitinib (PF299804,PF-00299804);DacoMitinib (PF299804, PF299);(2E)-N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-methoxy-6-quinazolinyl]-4-(1-piperidinyl)-2-butenamide Dacomitinib (PF299804, PF299) |
| CAS: | 1110813-31-4 |
| MF: | C24H25ClFN5O2 |
| MW: | 469.9390032 |
| EINECS: | -0 |
| Product Categories: | Anti-cancer&immunity;Inhibitors |
| Mol File: | 1110813-31-4.mol |
 |
|
| Dacomitinib (PF299804) Chemical Properties |
| density | 1.344 |
| CAS DataBase Reference | 1110813-31-4 |
| Safety Information |
| HS Code | 29335990 |
| Dacomitinib (PF299804) Usage And Synthesis |
| Description | Dacomitinib is a selective and irreversible inhibitor of EGFR. It is a drug candidate for the treatment of non-small cell lung carcinoma. It is current under the Phase III clinical trials. It also shows potential for the treatment of HER-2 amplified breast cancer cells lines that are resistant to trastuzumab and lapatinib. |
| Biological activity | Dacomitibib (Dacomitinib, PF299804) is an effective and irreversible pan-ErbB inhibitor that is most effective in EGFR, with an IC50 of 6 nM. It is also highly effective in NSCLCs that carries EGFR or ERBB2 mutants (against Gefitinib) and EGFR T790M mutant. Phase 2. Dacomitinib is taken orally once-daily. It is an irreversible inhibitor of HER-1 (EGFR), HER-2 and HER-4 tyrosine kinases. Dacomitinib targets multiple receptors of the HER pathway, whereas currently marketed HER-1 (EGFR) inhibitors for non-small cell lung cancer (NSCLC) target only one receptor in this pathway,developed by Pfizer. |
| Clinical Study | Dacomitinib has advanced to several Phase III clinical trials. The results of the first trials were disappointing, with a failure to meet the study goals, Additional Phase III trials are ongoing. Clinical evaluation of dacomitinib is ongoing in a number of clinical trials in patients with advanced NSCLC across lines of therapies and a range of histologies and molecular subtypes, such as EGFR and KRAS status. Additionally, there is an ongoing clinical trial evaluating dacomitinib in recurrent and/or metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN). |
| References | Ellis, Peter M,, et al. "Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. " Lancet Oncology 15.12(2014):1379. Kalous, O, et al. "Dacomitinib (PF-00299804), an irreversible Pan-HER inhibitor, inhibits proliferation of HER2-amplified breast cancer cell lines resistant to trastuzumab and lapatinib. " Molecular Cancer Therapeutics11.9(2012):1978. https://en.wikipedia.org/wiki/Dacomitinib |
| Uses | PF299804 is a potent, irreversible pan-ErbB inhibitor against ErbB1, ErbB2 and ErbB4 with IC50 of 6 nM, 45.7 nM and 73.7 nM, respectively. |
| Definition | ChEBI: A member of the class of quinazolines that is 7-methoxyquinazoline-4,6-diamine in which the amino group at position 4 is substituted by a 3-chloro-4-fluorophenyl group and the amino group at position 6 is substituted by an (E)-4-(piperidin 1-yl)but-2-enoyl group. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- SETV ASRV LLP
- DACOMITINIB 1110813-31-4 95-99%
- Inquiry
- 2024-09-11
-
VIP1年
- AKASH PHARMA EXPORTS
- Dacomitinib 99%
- Inquiry
- 2024-05-16
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
