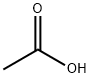
| Description |
Acetic acid is a colourless liquid or crystal with a sour, vinegar-like odour and is one of the simplest carboxylic acids and is an extensively used chemical reagent. Acetic acid has wide application as a laboratory reagent, in the production of cellulose acetate mainly for photographic film and polyvinyl acetate for wood glue, synthetic fibres, and fabric materials. Acetic acid has also been of large use as a descaling agent and acidity regulator in food industries. |
| Chemical Properties |
Clear colorless liquid |
| Uses |
Acetic acid is used as table vinegar, as preservative and as an intermediate in the chemical industry, e.g. acetate fibers, acetates, acetonitrile, pharmaceuticals, fragrances, softening agents, dyes (indigo) etc. Product Data Sheet |
| Uses |
It is used in aqueous and non-aqueous acid-base titrations. |
| Uses |
manufacture of various acetates, acetyl compounds, cellulose acetate, acetate rayon, plastics and rubber in tanning; as laundry sour; printing calico and dyeing silk; as acidulant and preservative in foods; solvent for gums, resins, volatile oils and many other substances. Widely used in commercial organic syntheses. Pharmaceutic aid (acidifier). |
| Definition |
ChEBI: A simple monocarboxylic acid containing two carbons. |
| Brand name |
Vosol (Carter-Wallace). |
| General Description |
A colorless aqueous solution. Smells like vinegar. Density 8.8 lb / gal. Corrosive to metals and tissue. |
| Air & Water Reactions |
Dilution with water releases some heat. |
| Reactivity Profile |
ACETIC ACID, [AQUEOUS SOLUTION] reacts exothermically with chemical bases. Subject to oxidation (with heating) by strong oxidizing agents. Dissolution in water moderates the chemical reactivity of acetic acid, A 5% solution of acetic acid is ordinary vinegar. Acetic acid forms explosive mixtures with p-xylene and air (Shraer, B.I. 1970. Khim. Prom. 46(10):747-750.). |
| Hazard |
Corrosive; exposure of small amounts can severely erode the lining of the gastrointestinal tract; may cause vomiting, diarrhea, bloody feces and urine; cardiovascular failure and death. |
| Health Hazard |
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
| Fire Hazard |
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. |
| Agricultural Uses |
Herbicide, Fungicide, Microbiocide; Metabolite, Veterinary Medicine: A herbicide used to control grasses, woody plants and broad-leaf weeds on hard surface and in areas where crops are not normally grown; as a veterinary medicine. |
| Trade name |
ACETUM®; ACI-JEL®; ECOCLEAR®; NATURAL WEED SPRAY® No. One; VOSOL® |
| Safety Profile |
A human poison by an unspecified route. Moderately toxic by various routes. A severe eye and skin irritant. Can cause burns, lachrymation, and conjunctivitis. Human systemic effects by ingestion: changes in the esophagus, ulceration, or bleeding from the small and large intestines. Human systemic irritant effects and mucous membrane irritant. Experimental reproductive effects. Mutation data reported. A common air contaminant. A flammable liquid. A fire and explosion hazard when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use CO2, dry chemical, alcohol foam, foam and mist. When heated to decomposition it emits irritating fumes. Potentially explosive reaction with 5- azidotetrazole, bromine pentafluoride, chromium trioxide, hydrogen peroxide, potassium permanganate, sodium peroxide, and phosphorus trichloride. Potentially violent reactions with acetaldehyde and acetic anhydride. Ignites on contact with potassium tert-butoxide. Incompatible with chromic acid, nitric acid, 2-amino-ethanol, NH4NO3, ClF3, chlorosulfonic acid, (O3 + diallyl methyl carbinol), ethplenediamine, ethylene imine, (HNO3 + acetone), oleum, HClO4, permanganates, P(OCN)3, KOH, NaOH, xylene |
| Purification Methods |
Usual impurities are traces of acetaldehyde and other oxidisable substances and water. (Glacial acetic acid is very hygroscopic. The presence of 0.1% water lowers its m by 0.2o.) Purify it by adding some acetic anhydride to react with water present, heat it for 1hour to just below boiling in the presence of 2g CrO3 per 100mL and then fractionally distil it [Orton & Bradfield J Chem Soc 960 1924, Orton & Bradfield J Chem Soc 983 1927]. Instead of CrO3, use 2-5% (w/w) of KMnO4, and boil under reflux for 2-6hours. Traces of water have been removed by refluxing with tetraacetyl diborate (prepared by warming 1 part of boric acid with 5 parts (w/w) of acetic anhydride at 60o, cooling, and filtering off, followed by distillation [Eichelberger & La Mer J Am Chem Soc 55 3633 1933]. Refluxing with acetic anhydride in the presence of 0.2g % of 2-naphthalenesulfonic acid as catalyst has also been used [Orton & Bradfield J Chem Soc 983 1927]. Other suitable drying agents include anhydrous CuSO4 and chromium triacetate: P2O5 converts some acetic acid to the anhydride. Azeotropic removal of water by distillation with thiophene-free *benzene or with butyl acetate has been used [Birdwhistell & Griswold J Am Chem Soc 77 873 1955]. An alternative purification uses fractional freezing. [Beilstein 2 H 96, 2 IV 94.] Rapid procedure: Add 5% acetic anhydride, and 2% of CrO3. Reflux and fractionally distil. |