

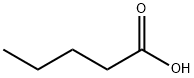
Valeric acid
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameValeric acid
- CAS No.109-52-4
- EINECS No.203-677-2
- MFC5H10O2
- MW102.13
- InChIKeyNQPDZGIKBAWPEJ-UHFFFAOYSA-N
- AppearanceLiquidClear colorless to pale yellow
- density 0.939 g/mL at 25 °C(lit.)
- Boiling point 110-111 °C10 mm Hg(lit.)
- Melting point −20-−18 °C(lit.)
- Water Solubility 40 g/L (20 ºC)
- storage temp. Store below +30°C.
AD68
| Valeric acid Basic information |
| Product Name: | Valeric acid |
| Synonyms: | N-PENTANOIC ACID;N-VALERIC ACID;PROPYLACETIC ACID;PENTANOIC-2,2-D2 ACID;PENTANOIC ACID;PENTANE ACID;RARECHEM AL BO 0179;VALERIC ACID |
| CAS: | 109-52-4 |
| MF: | C5H10O2 |
| MW: | 102.13 |
| EINECS: | 203-677-2 |
| Product Categories: | API intermediates;Alkylcarboxylic Acids;Monofunctional & alpha,omega-Bifunctional Alkanes;Monofunctional Alkanes;Aliphatics;Carboxylic Acids;Biochemicals and Reagents;Building Blocks;C1 to C5;Carbonyl Compounds;Carboxylic Acids;Chemical Synthesis;Fatty Acids and conjugates;Fatty Acyls;Ginkgo biloba;Humulus lupulus (Hops);Lavandula angustifolia (Lavendar tea);Lipids;Nutrition Research;Ocimum basilicum (Basil);Organic Building Blocks;Panax ginseng;Phytochemicals by Plant (Food/Spice/Herb);Sambucus nigra (Elderberry);Straight Chain Fatty Acids;Vaccinium macrocarpon (Cranberry);Flavour Enhancer and Aromas;Benzimidazoles |
| Mol File: | 109-52-4.mol |
 |
|
| Valeric acid Chemical Properties |
| Melting point | −20-−18 °C(lit.) |
| Boiling point | 110-111 °C10 mm Hg(lit.) |
| density | 0.939 g/mL at 25 °C(lit.) |
| vapor density | 3.5 (vs air) |
| vapor pressure | 0.15 mm Hg ( 20 °C) |
| refractive index | n20/D 1.408(lit.) |
| FEMA | 3101 | VALERIC ACID |
| Fp | 192 °F |
| storage temp. | Store below +30°C. |
| solubility | 40g/l |
| pka | 4.84(at 25℃) |
| form | Liquid |
| color | Clear colorless to pale yellow |
| PH | 2.7 (40g/l, H2O, 20℃) |
| explosive limit | 1.8-7.3%(V) |
| Water Solubility | 40 g/L (20 ºC) |
| Merck | 14,9904 |
| BRN | 969454 |
| CAS DataBase Reference | 109-52-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Pentanoic acid(109-52-4) |
| EPA Substance Registry System | Pentanoic acid(109-52-4) |
| Safety Information |
| Hazard Codes | C |
| Risk Statements | 34-52/53 |
| Safety Statements | 26-36-45-61 |
| RIDADR | UN 3265 8/PG 3 |
| WGK Germany | 1 |
| RTECS | YV6100000 |
| F | 13 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 29156090 |
| Hazardous Substances Data | 109-52-4(Hazardous Substances Data) |
| Toxicity | LD50 i.v. in mice: 1290 ±53 mg/kg (Or, Wretlind) |
| MSDS Information |
| Provider | Language |
|---|---|
| 1-Butanecarboxylic acid | English |
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| Valeric acid Usage And Synthesis |
| Description | Valeric acid (also pentanoic acid) is a straight chain alkyl carboxylic acid. Valeric acid is mainly used for the synthesis of its esters used in foods, perfume, and cosmetics. Valeric acid is used as an intermediate for applications including ester type lubricants (in aviation turbine oils, fire-resistance hydraulic fluids, and refrigerator oils), plasticizers (dipropyl heptyl phthalate), vinyl stabilizers, and specialty chemicals. It is also used as an odorant in pesticide formulations for use on crops. |
| References | [1] http://www.chemicalland21.com [2] Patent CA 2496307 C: High viscosity synthetic ester lubricant base stock [3] https://www.icis.com [4] Christopher G. Morris (1992) Academic Press Dictionary of Science and Technology |
| Chemical Properties | Colorless liquid; penetrating odor and taste. Soluble in water, alcohol and ether. Undergoes reactions typical of normal monobasic organic acids. Combustible. |
| Uses | valeric acid is obtained from valerian extract, which is considered skin conditioning. |
| Uses | Sex attractant of the sugar beet wireworm, Limonius californicus.1 |
| Definition | ChEBI: A straight-chain saturated fatty acid containing five carbon atoms. |
| General Description | A colorless liquid with a penetrating unpleasant odor. Density 0.94 g / cm3. Freezing point -93.2°F (-34°C). Boiling point 365.7°F (185.4°C). Flash point 192°F (88.9°F). Corrosive to metals and tissue. |
| Air & Water Reactions | Water soluble. |
| Reactivity Profile | Valeric acid is a carboxylic acid. Exothermically neutralizes bases, both organic and inorganic, producing water and a salt. Can react with active metals to form gaseous hydrogen and a metal salt. Reacts with cyanide salts to generate gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by reaction with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Reacts with sulfites, nitrites, thiosulfates and dithionites to generate flammable and/or toxic gases and heat. Reacts with carbonates and bicarbonates to generate a harmless gas (carbon dioxide) but still heat. Can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. May initiate polymerization reactions. May catalyze (increase the rate of) chemical reactions. |
| Health Hazard | Corrosive. Very destructive to tissues of the mucous membranes, upper respiratory tract, eyes, and skin. Symptoms may include burning sensation, coughing, wheezing, laryngitis, shortness of breath, nausea and vomiting. Extremely destructive to skin. May be absorbed through the skin. |
| Fire Hazard | Special Hazards of Combustion Products: Irritating vapors and toxic gases, such as carbon dioxide and carbon monoxide, may be formed when involved in fire. |
| Safety Profile | Moderately toxic by ingestion, intravenous, and subcutaneous routes. Mildly toxic by inhalation. A corrosive irritant to skin, eyes, and mucous membranes. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. Used in perfumes. |
| Purification Methods | Water is removed from the acid by distillation using a Vigreux column (p 11), until the boiling point reaches 183o. A few crystals of KMnO4 are added, and after refluxing, the distillation is continued. [Andrews & Keefer J Am Chem Soc 83 3708 1961, Beilstein 2 H 299, 2 I 130, 2 II 263, 2 III 663, 2 IV 868.] |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Varanous Labs Pvt Ltd
- 109-52-4 98%
- Inquiry
- 2023-12-26
-
VIP1年
- JSK Chemicals
- 109-52-4 Valeric acid, 98% 99%
- Inquiry
- 2023-12-22
-
VIP1年
- SS Reagents and Chemicals
- 2067-33-6 99%
- Inquiry
- 2024-10-24
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
