

Dichlorodimethylsilane
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1kg
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameDichlorodimethylsilane
- CAS No.75-78-5
- EINECS No.200-901-0
- MFC2H6Cl2Si
- MW129.06
- InChIKeyLIKFHECYJZWXFJ-UHFFFAOYSA-N
- Appearanceliquidcolorless
- Water Solubility reacts
- Melting point -76 °C
- storage temp. Store below +30°C.
- density 1.333 g/mL at 20 °C
- Boiling point 70 °C(lit.)
AD68
| Dichlorodimethylsilane Basic information |
| Product Name: | Dichlorodimethylsilane |
| Synonyms: | (CH3)2SiCl2;dichlorodimethyl-silan;Dichlorodimethylsilicon;dimethyldichlorosilicane;Dimethyl-dichlorsilan;dimethyl-dichlorsilan(czech);Dimethyldichlorsilane;dimethylsilanedichloride |
| CAS: | 75-78-5 |
| MF: | C2H6Cl2Si |
| MW: | 129.06 |
| EINECS: | 200-901-0 |
| Product Categories: | Chloro;Silicon Compounds;Organics;Dichlorosilanes;Dichlorosilanes (for Polysilanes);Functional Materials;Reagent for High-Performance Polymer Research;Si (Classes of Silicon Compounds);Si-Cl Compounds;Silicon Compounds (for Synthesis);Synthetic Organic Chemistry;Chloro Silanes;organosilicon compounds;Silicone Series |
| Mol File: | 75-78-5.mol |
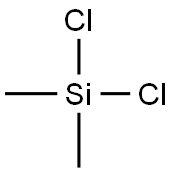 |
|
| Dichlorodimethylsilane Chemical Properties |
| Melting point | -76 °C |
| Boiling point | 70 °C(lit.) |
| density | 1.333 g/mL at 20 °C |
| refractive index | n20/D 1.500 |
| Fp | 3 °F |
| storage temp. | Store at 0-5°C |
| form | liquid |
| color | colorless |
| explosive limit | 1.75-48.5%(V) |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| BRN | 605287 |
| Stability: | Stable. Reacts violently with water and alcohols. Highly flammable. Incompatible with strong oxidizing agents, water, alcohols, caustics, ammonia. |
| CAS DataBase Reference | 75-78-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, dichlorodimethyl-(75-78-5) |
| EPA Substance Registry System | Silane, dichlorodimethyl-(75-78-5) |
| Safety Information |
| Hazard Codes | Xn,Xi,F,N,C |
| Risk Statements | 20-59-36/37/38-11-67-65-63-48/20-38-20/21-50/53-37-35-20/22-14-34 |
| Safety Statements | 60-61-62-36/37-59-45-36/37/39-26-16-7/9-2 |
| RIDADR | UN 2924 3/PG 2 |
| WGK Germany | 3 |
| RTECS | VV3150000 |
| F | 3-10-19-21 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | II |
| HS Code | 29310095 |
| Hazardous Substances Data | 75-78-5(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| Dichlorodimethylsilane | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Dichlorodimethylsilane Usage And Synthesis |
| Description |
Dichlorodimethylsilane is a tetrahedral, organosilicon compound with the molecular formula Si(CH3)2Cl2. The compound is a colorless liquid at room temperature, has a pungent odor and readily reacts with water to form both cyclic and linear Si-O chains. On an industrial scale, dichlorodimethylsilane is manufactured as the main precursor to polysilane and dimethylsilicone compounds. |
| History |
James Crafts and Charles Friedel are American chemists who first reported organosilicon compounds in 1863 by synthesizing tetraethylsilane from silicon tetrachloride and diethylzinc. Nevertheless, major progress in organosilicon chemistry occurred when Fredrick Kipping and his students reacted tetrachloride with Grignard reagents to produce diorganodichlorosilanes (R2SiCl2), which they used for their experiments. The dpemand for silicones increased in the 1930s as many aircraft companies needed better insulators for sealing materials and electric motors for aircraft engines; therefore, there was need for efficient synthesis of dichlorodimethylsilane. |
| Preparation |
Dichlorodimethylsilane is prepared by passing methyl chloride through a heated tube packed with copper (I) chloride and ground silicon using Cu2O as the catalyst. Methyl chloride is the passed through a reactor to produce dichlorodimethylsilane. |
| Applications |
Dichlorodimethylsilane is majorly used in the production of silicones. Moreover, it is utilized in the synthesis of polysilanes, which are the main precursors to silicon carbide. Dichlorodimethylsilane can be used to coat glass to prevent the adsorption of micro-particles. |
| Chemical Properties | Colorless to brown liquid |
| Uses | Dichlorodimethylsilicon is a organosilicon compound and is the precursor to dimethylsilicone and polysilane compounds. |
| General Description | A colorless fuming liquid with a pungent odor. Flash point 16°F. Vapor and liquid may cause burns. Denser than water. Vapors heavier than air. |
| Reactivity Profile | Chlorosilanes, such as Dichlorodimethylsilane, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases. |
| Health Hazard | Inhalation irritates mucous membranes. Severe gastrointestinal damage may occur. Vapors cause severe eye and lung injury. Upon short contact, second and third degree burns may occur. |
| Fire Hazard | Vapor may explode if ignited in an enclosed area. Reacts vigorously with water to generate hydrogen chloride. Hydrogen chloride and phosgene gases may be formed upon heating or in fire. Runoff to sewer may create fire or explosion hazard. |
| Safety Profile | Poison by ingestion and intraperitoneal routes. Moderately toxic by inhalation. A skin and severe eye irritant. Violent reaction on contact with water. When heated to decomposition it emits toxic fumes of Cl-. See also CHLOROSILANES. |
| Purification Methods | Other impurities are chlorinated silanes and methylsilanes. Fractionate it through a 3/8in diameter 7ft Stedman column (p 11) rated at 100 theoretical plates at almost total reflux. See purification of MeSiCl2. Solutions in heptane, 1,1,1-trichloroethane or 1-chloronaphthalene are used for the silanization of glassware and pipettes. [Sauer & Hadsell J Am Chem Soc 70 3590 1948, Beilstein 4 IV 4110.] |
Company Profile Introduction
Recommended supplier
-
VIP1年
- Otto Chemie Pvt Ltd
- Dichlorodimethylsilane 98%
- Inquiry
- 2024-01-04
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
