

Torezolid
| Price | USD7.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:1000KG
- Time:2019-07-06
Product Details
- Product Name Torezolid
- CAS No. 856866-72-3
- EINECS No.815-370-6
- MFC17H15FN6O3
- MW370.34
- AppearanceSolidWhite to Off-White
- density 1.57
- Melting point 201 °C
- Boiling point 614.5±65.0 °C(Predicted)
- storage temp. Refrigerator
JD607
| Product Name: | Torezolid |
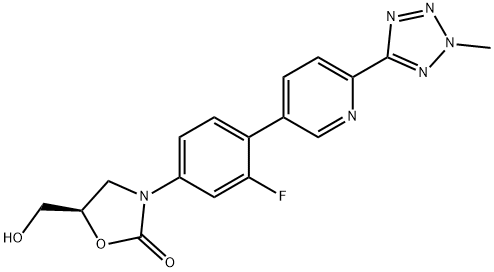
| Synonyms: | 3-[3-Fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-5-(hydroxymethyl)-2-oxazolidinone;Torezolid;Tedizolid;DA 7157;DA-7157;-3-(4-(2-(2-Methyltetrazol-5-yl)pyridine-5-yl)-3-fluorophenyl)-5-hydroxyMethyl oxazolidin-2-one;Tedizolid (TR-701);(R)-3-(3-fluoro-4-(6-(2-Methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenyl)-5-(hydroxyMethyl)oxazolidin-2-one |
| CAS: | 856866-72-3 |
| MF: | C17H15FN6O3 |
| MW: | 370.3378032 |
| EINECS: | 1592732-453-0 |
| Product Categories: | Tedizolid intermediates;Inhibitors |
| Mol File: | 856866-72-3.mol |
 |
|
| Torezolid Chemical Properties |
| density | 1.57 |
| Safety Information |
| Torezolid Usage And Synthesis |
| Novel antibacterial drugs | Tedizolid belongs to the second generation oxazolidinone antibiotics, being an analog of linezolid as well as a kind of protein synthesis inhibitors, acting on the bacterial ribosomal 50S subunit and causing inhibition of bacterial protein synthesis. Compared with linezolid, the efficacy of the two is basically the same, but linezolid required to be taken for 2 times a day and for 10 continuous days while Tedizolid only takes 1 time daily and continuous taking for 6 days. On June 20, 2014, the US FDA had approved a new antimicrobial drug Tedizolid (trade name: Sivextro) for the treatment of skin infections in adult patients. Sivextro is approved for the treatment of acute bacterial skin and skin infections caused by certain sensitive bacteria such as Staphylococcus aureus (including methicillin-resistant strains and methicillin-sensitive strains), various streptococci and Enterococcus faecalis (ABSSSI). Sivextro is administered intravenously and orally. Tedizolid phosphate is a second-generation oxazolidinone antibiotic developed by Dong-A Pharmaceutical, licensed to Cubist Pharmaceuticals and Bayer for commercial development. Sivextro is designed for the treatment of serious or life-threatening infections, and its listing applications are eligible for Qualified Infectious Disease Products (QIDP) and have obtained the approve of FDA for accelerated review. Sivextro's QIDP eligibility gives the drug exclusive five-year market exclusivity, in addition to its market exclusivity under the Food, Drug, and Cosmetic Act. The safety and efficacy of Sivextro were evaluated in two clinical trials involving 1315 ABSSSI adult patients. Subjects were randomized to Sivextro or another antibiotic linezolid approved for the treatment of acute bacterial skin and skin structure infections (ABSSSI). The results showed that its clinical efficacy is equivalent to linezolid. However, it will cause less gastrointestinal adverse reactions and thrombocytopenia adverse reactions than linezolid. The incidence of drug resistance is also lower. Tedizolid has been shown to be more tolerant than vancomycin. The most common side effects identified in clinical trials include nausea, headache, diarrhea, vomiting and dizziness. The safety and efficacy of Sivextro have not been evaluated in patients with reduced white blood cell levels (neutropenia), so alternative therapies should be considered for these patients. Sivextro is marketed by Cubist Pharmaceuticals, Inc., based in Lexington, Massachusetts. Status of intellectual property: Compound patent ZL200480037612.2, protection period 2024.12.17 expires. This information was edited by Xiao Nan from Chemicalbook (2015-08-14). |
| Indications | Tedizolid phosphate is an oxazolidinone compound used in the treatment of acute bacterial skin and skin structure infections caused by the following gram-positive bacteria-sensitive strains: Staphylococcus aureus (including Methicillin-resistant and methicillin-susceptible strains), pyogenic streptococci, Streptococcus lactis, Streptococcus angustifolia (including Angina, Streptococcus intermedius and Streptococcus constellation), and Enterococcus faecalis. |
| State of Intellectual Property | (A) Administrative protection, new drug protection and new drug monitoring period On August 9, 2013, Bayer Pharmaceuticals has filed a new drug application with CFDA. (B) Domestic patent 1. Patented compounds East Asia Pharmaceutical has applied for the compound patent in China under the application number is ZL200480037612.2. The date of application is December 17, 2004; the protection period will expire at 2024.12.17. Three subsequent divisional applications were further filed with the application numbers being 201010508824.1 (preparation), 201110304983.4, 201210155386.4. On October 9, 2009, the Teresius Therapeutics Company has applied for a preparation method patent with the patent number of 200980140144.4. 2. Crystal patent On February 3, 2010, Teulius Therapeutics applied for a crystalline form patent of free acid with the application number of 201080014363.0. Form I of the disclosed free acid has the advantage that it is more stable than the disodium salt and has no moisture absorption. 3. Formulation Composition Patents The above-mentioned patent 200480037612.2 and its divisional application patent have disclosed the Tedizolid powder, tablet, capsule and injection. The above-mentioned patent 201080014363.0 have disclosed the prescription of Tedizolid tablets and freeze-dried preparations. |
| Uses | Tedizolid, known as TR-700, is an oral and i.v administered intracellular antibacterial drug. |
| Definition | ChEBI: A member of the class of pyridines that is pyridine which is substituted by a 2-methyl-2H-tetrazol-5-yl group at position 2 and by a 2-fluoro-4-[(5R)-5-(hydroxymethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl group at position 5 It is used as its phosphate pro-drug used for the treatment of acute bacterial skin and skin structure infections caused by certain susceptible bacteria, including Staphylococcus aureus (including methicillin-resistant strains (MRSA) and meth cillin-susceptible strains), various Streptococcus species, and Enterococcus faecalis. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Honour Lab Limited
- 856866-72-3 98%
- Inquiry
- 2024-01-08
-
VIP1年
- Lupin Ltd
- 856866-72-3 Tedizolid 99%
- Inquiry
- 2024-01-05
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
