

Olsalazine sodium
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameOlsalazine sodium
- CAS No.6054-98-4
- EINECS No.227-975-7
- MFC14H11N2NaO6
- MW326.24
- AppearanceneatLight Yellow to Yellow
- Melting point 240 °C
- storage temp. Inert atmosphere,Store in freezer, under -20°C
AD68
| Olsalazine sodium Basic information |
| Product Name: | Olsalazine sodium |
| Synonyms: | 3,3’-azobis[6-hydroxy-benzoicacidisodiumsalt;Benzoicacid,3,3’-azobis[6-hydroxy-,disodiumsalt;olsalazinedisodiumsalt;OLSALAZINE DISODIUM;OLSALAZINE SODIUM;disodium 5,5'-azodisalicylate;Acid mordant Yellow 5;Chrome Yellow AS |
| CAS: | 6054-98-4 |
| MF: | C14H8N2O6.2Na |
| MW: | 346.2 |
| EINECS: | 227-975-7 |
| Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;Aromatics;Pharmaceutical intermediate;API;AZODISAL SODIUM |
| Mol File: | 6054-98-4.mol |
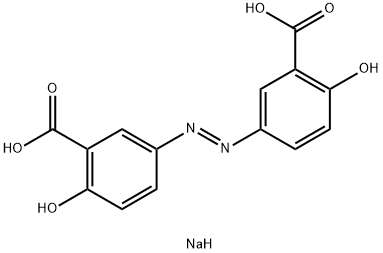 |
|
| Olsalazine sodium Chemical Properties |
| Melting point | 240 °C |
| storage temp. | -20°C Freezer |
| CAS DataBase Reference | 6054-98-4(CAS DataBase Reference) |
| EPA Substance Registry System | Benzoic acid, 3,3'-azobis[6-hydroxy-, disodium salt(6054-98-4) |
| Safety Information |
| Olsalazine sodium Usage And Synthesis |
| Treatment of acute and chronic colitis | Olsalazine sodium is a drug developed by Pharmacia A B Co., Switzerland, to treat acute and chronic colitis. It was first listed in Denmark in 1989. It has been collected and recordedby the European Pharmacopoeia 7 edition and the national drug standard WS1- (X-349) -2004Z. Orsalazine sodium is a precursor drug consisting of two azo bonds linked to 5- amino salicylic acid. The bacteria in the colon break up the diazo bond and release 5- amino salicylic acid. 5- amino salicylic acid acts on colitis mucosa, inhibits the formation of prostaglandins, leukotrienes and other inflammatory factors, and reduces membrane permeability, and plays a role in the treatment of ulcerative colitis. This product weakens the absorption of nitrogen, sodium and water in the ileum and colonic mucosa, which is transformed into secretion in human body. Patients with ulcerative colitis take 1g daily, and the whole intestinal transit time is shortened by 40%. If the healthy subjects are given oral administration of 2g once, It has no effect on the complex frequency of migration of the small intestine. But it can cause diarrhea in 30% of the users and increase the diarrhea of the patients with intestinal inflammation. The bioavailability of the whole body is extremely low. The absorption rate of the oral dose is less than 5%. After absorption, about 10% doses will be conjugated to orsalazine sulfate, t1/2 about 1H and conjugated half life is about 7 days. 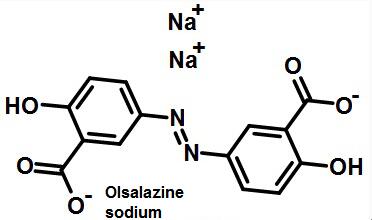 Figure 1 The structural formula of oralalazine sodium |
| Adverse reaction | The most common side effect is diarrhoea, the incidence of which is 17%. Usually, the difference between diarrhea and intestinal inflammation is that the excreta has high water content without blood, and is related to the dosage. It usually occurs when the treatment begins or the dose increases. Reduction the dose of this product or combined use with Loperamide, diarrhea will be controlled. Other side effects include headache, nausea, abdominal pain, rash, dizziness and joint pain. Most of the patients who are allergic or intolerant to sulfasalazine are resistant to this product. The male sterility treated with sulfasalazine improved after using this product. |
| Chemical Properties | Yellow Crystalline Powder |
| Uses | Dimer of Mesalamine. It is an anti-inflammatorydrug used in the treatment of inflammatory bowel disease and ulcerative colitis |
| Uses | Dimer of Mesalazine (M258100), an anti-inflammatory drug used in the treatment of inflammatory bowel disease and ulcerative colitis. |
| Definition | ChEBI: An organic sodium salt that is the disodium salt of 3,3'-azobis(6-hydroxybenzoic acid) (olsalazine). Effective in the treatment of inflammatory bowel disease and ulcerative colitis. Mechanism of action unknown, but appears to be topical |
| Uses | Mordant dye for wool. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Hetero Drugs Limited
- 6054-98-4 Olsalazine sodium 98%
- Inquiry
- 2024-01-08
-
VIP1年
- Allmpus Laboratories Pvt Ltd
- FUSIDIC ACID SODIUM SALT / SODIUM FUSIDATE / FUSIDIC ACID SODIUM SALT 95.65
- Inquiry
- 2024-09-14
-
VIP1年
- Nivika Chemo Pharma Pvt Ltd
- Olsalazine 99%
- Inquiry
- 2024-01-02
-
VIP1年
- Varanous Labs Pvt Ltd
- 15722-48-2 Olsalazine 98%
- Inquiry
- 2023-12-26
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
