

Canagliflozin
| Price | USD7.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product Name Canagliflozin
- CAS No. 842133-18-0
- EINECS No.695-192-1
- MFC24H25FO5S
- MW444.52
- AppearancesolidWhite
- density 1.364
- Boiling point 642.9±55.0 °C(Predicted)
- storage temp. -20°C
- Melting point 68 - 72°C
JD607
| Product Name: | Canagliflozin |
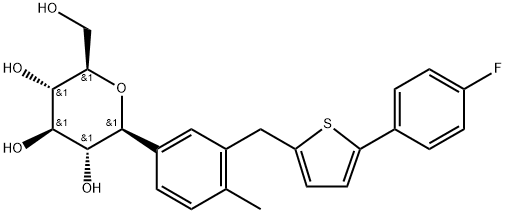
| Synonyms: | TA 7284;Canaglifozion;D-Glucitol, 1,5-anhydro-1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]Methyl]-4-Methylphenyl]-, (1S)-;Cango coluMn net;Canagliflozin WS;Canagliflozin(TA-7284);(2S,3R,4R,5S,6R)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3,4,5-triol;Canaglifozin |
| CAS: | 842133-18-0 |
| MF: | C24H25FO5S |
| MW: | 444.5157032 |
| EINECS: | 1308068-626-2 |
| Product Categories: | Aromatics;Heterocycles;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds;Other APIs;Stable Isotopes |
| Mol File: | 842133-18-0.mol |
 |
|
| Canagliflozin Chemical Properties |
| density | 1.364 |
| Safety Information |
| Canagliflozin Usage And Synthesis |
| Abstract | Canagliflozin(Invokana) is an oral diabetes medicine that helps control blood sugar levels. It works by helping the kidneys get rid of glucose from your bloodstream. Invokana is used together with diet and exercise to treat type 2 diabetes. To mention the advantage, this drug helps patients to reduce blood sugars without increasing the likelihood of gaining weight as long as a healthy, balanced diet is followed and regular exercise taken. |
| Oral diabetes drugs | Canagliflozin is an Inhibitor drug for SGLT2. It can lower blood glucose levels by expelling the glucose from the body through the kidneys after its decomposition. In addition, Canagliflozin has a broad prospect due to its significant antiobesity effects and few hypoglycemic events. According to the results of 2 phase III clinical trials on June 9th, 2012, Canagliflozin, which is an Experimental type 2 diabetes drug of JNJ is better than Januvia of Merck or an older drug called glimepiride. Meanwhile, Canagliflozin has a more significant antiobesity effect. Compared with glimepiride, it causes less hypoglycemic events. On January 11th, 2013, Janssen Pharmaceutical of JNJ announced that its diabetes drug canagliflozin (trade name INVOKANA?)was recommended and approved by the committee of experts of FDA by the vote of 10 to 5 to treat adult patients with type 2 diabetes. On march 29th, 2013, canagliflozin (trade name INVOKANA, Janssen Pharmaceutical China of JNJ)was approved by the US Food and Drug Administration (FDA) to control blood glucose of adult patients with type 2 diabetes. On December 25th, 2013, Janssen Pharmaceutical of JNJ announced that new diabetes drug INVOKANA(canagliflozin)was approved by European Commission(EC)to treat adult patients with type 2 diabetes and improve glucose control. In September, 2014, Canagliflozin Won the positive Suggestions for recommend approval of Committee for Medicinal Products for Human Use (CHMP) of European Medicines Agency (EMA). In addition, Canagliflozin has been approved by FDA in March this year, and approved by Australia rencently. Canagliflozin is a once daily oral diabetes drug, which works by inhibiting sodium glucose co-transporter 2 (SGLT2)as a new drug. It can lower blood glucose levels by interdicting the blood glucose reabsorption of the kidneys and increasing the excretion of blood glucose in urine. Compared with non-diabetic population, the kidneys of adult patients with type 2 diabetes absorb a large number of glucose into the blood stream, which will push up the blood glucose levels. Canagliflozin is one of the most promising drug candidates of JNJ. The department of Janssen Pharmaceutical of JNJ has the rights to sell this drug in North America, South America, Europe, Middle East, Africa, Australia, New Zealand and some Asian countries. |
| Clinical study | Canagliflozin is the first FDA approved inhibitor for SGLT2 and used to treat adult patients with type 2 diabetes. As a kind of glucose transporter, SGLT has two subtypes of SGLT1and SGLT2, which are distributed in the intestinal mucosa and renal tubular respectively, and able to transport glucose into the blood. Canagliflozin can inhibit SLCT2 and, interdict the reabsorption of glucose in Renal tubular and increase the excretion of blood glucose in urine. Thus, the blood glucose levels can be lowered. Glucose was passed into the urine through the kidneys, accompanied by renal impairment, symptomatic hypotension, fungal infections and other side effects. 9 clinical trials involving 10285 patients have demonstrated the safety and efficacy of Invokana, either used alone or in combination with other hypoglycemic agents. In a double-blind controlled trial for 26 weeks, 584 adult patients with type 2 diabetes were divided into three groups, namely 100mg Canagliflozin group, 300mg Canagliflozin group and placebo group. Compared with placebo group, the HbA1c of the 100mg Canagliflozin group and 300mg Canagliflozin group was lowed extra 0.91% and 1.16% respectively. |
| How it works | Invokana is in a class of drugs called sodium-glucose transport protein 2 (or SGLT2) inhibitors, which drugs work by increasing the amount of glucose that gets passed out in the urine. When blood passes through the kidneys, the kidneys filter glucose out of the blood and the SGLT proteins then help reabsorb glucose back into the blood. SGLT2 proteins are responsible for 90% of the glucose that is reabsorbed, so by blocking the action these proteins, less glucose is reabsorbed and so more glucose is excreted via the urine. |
| Invokana tables | Invokana is supplied as film-coated tablets for oral administration, containing 102 and 306 mg of canagliflozin in each tablet strength, corresponding to 100 mg and 300 mg of canagliflozin (anhydrous), respectively. Inactive ingredients of the core tablet are croscarmellose sodium, hydroxypropyl cellulose, lactose anhydrous, magnesium stearate, and microcrystalline cellulose. The magnesium stearate is vegetable-sourced. The tablets are finished with a commercially available film-coating consisting of the following excipients: polyvinyl alcohol (partially hydrolyzed), titanium dioxide, macrogol/PEG, talc, and iron oxide yellow, E172 (100 mg tablet only). |
| Taboo | You should not use Invokana if you have severe kidney disease (or if you are on dialysis). Invokana is not for treating type 1 diabetes. |
| References | https://www.drugs.com/cdi/canagliflozin.html |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Sibram Pharmaceutical
- Canagliflozin 99%
- Inquiry
- 2024-03-16
-
VIP1年
- Turtle Pharma Private Limited
- Canagliflozin 98%
- Inquiry
- 2024-03-15
-
VIP1年
- Vijaya Pharma And Life Science
- Canagliflozin 98%
- Inquiry
- 2024-03-13
-
VIP1年
- Mylan Laboratories Ltd
- 842133-18-0 98%
- Inquiry
- 2024-03-06
-
VIP1年
- Progress Life Sciences Pvt Ltd
- 842133-18-0 Canagliflozin 98%
- Inquiry
- 2024-03-04
-
VIP1年
- BDR Pharmaceuticals International Pvt Ltd
- Canagliflozin 842133-18-0 98%
- Inquiry
- 2024-02-24
-
VIP1年
- Indoco Remedies Ltd
- Canagliflozin 98%
- Inquiry
- 2024-01-23
-
VIP1年
- Hikal Ltd.
- 842133-18-0 98%
- Inquiry
- 2024-01-08
-
VIP1年
- Macleods Pharmaceuticals Limited
- 842133-18-0 Canagliflozin 98%
- Inquiry
- 2024-01-08
-
VIP1年
- Honour Lab Limited
- Canagliflozin 842133-18-0 98%
- Inquiry
- 2024-01-08
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
