

Amstat
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product Name Amstat
- CAS No. 1197-18-8
- EINECS No.214-818-2
- MFC8H15NO2
- MW157.21
- InChIKeyGYDJEQRTZSCIOI-LJGSYFOKSA-N
- AppearanceCrystalline PowderWhite
- storage temp. 2-8°C
- Water Solubility 1g/6ml
- Melting point >300 °C (lit.)
- Boiling point 281.88°C (rough estimate)
- density 1.0806 (rough estimate)
AD68
| Amstat Basic information |
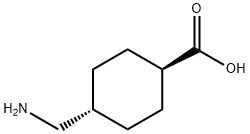
| Product Name: | Amstat |
| Synonyms: | TIMTEC-BB SBB006715;TRANS-4-AMINOMETHYL-1-CYCLOHEXANECARBOXYLIC ACID;trans-4-aminomethylcyclohexane-1-carboxylate;TRANS-4-(AMINOMETHYL)CYCLOHEXANECARBOXYLIC ACID;TRANEXAMIC ACID;amstat;amcha;amikapron |
| CAS: | 1197-18-8 |
| MF: | C8H15NO2 |
| MW: | 157.21 |
| EINECS: | 214-818-2 |
| Product Categories: | 4-Substituted Cyclohexanecarboxylic Acids;API's;Amines;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;Building Blocks;C8;Carbonyl Compounds;Carboxylic Acids;Chemical Synthesis;Organic Building Blocks;API;CYKLOKAPRON;Cosmetics;Hemostatic;Pharmaceutical raw materials;Pharmaceutical intermediates |
| Mol File: | 1197-18-8.mol |
 |
|
| Amstat Chemical Properties |
| Melting point | >300 °C(lit.) |
| Boiling point | 281.88°C (rough estimate) |
| density | 1.0806 (rough estimate) |
| refractive index | 1.4186 (estimate) |
| storage temp. | Store at 0°C |
| form | Crystalline Powder |
| color | White |
| Water Solubility | 1g/6ml |
| Merck | 14,9569 |
| BRN | 2207452 |
| InChIKey | GYDJEQRTZSCIOI-LJGSYFOKSA-N |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36-37/39 |
| WGK Germany | 2 |
| RTECS | GU8400000 |
| HazardClass | IRRITANT |
| Toxicity | LD50 in mice, rats (mg/kg): 1500, 1200 i.v. (Melander) |
| Amstat Usage And Synthesis |
| Properties | Amstat is an antifibrinolytic drug, commonly called Tranexamic acid. Tranexamic acid is a white crystalline powder. It is freely soluble in water and in glacial acetic acid and is very slightly soluble in ethanol and practically insoluble in ether. | ||||||||
| Coagulation and hemostasis drugs | Tranexamic acid is a derivative of aminomethylbenzoic acid, and a kind of antifibrinolytic drugs to stop bleeding. The hemostasis mechanism of tranexamic acid is similar to aminocaproic acid and aminomethylbenzoic acid, but the effect is stronger. The strength is 7 to 10 times of aminocaproic acid, 2 times of aminomethylbenzoic acid, but toxicity is similar. The chemical structure of tranexamic acid is similar to lysine, competitive inhibition of plasmin original in fibrin adsorption, to prevent their activation, protection fiber protein not to degrade by plasmin and dissolve, eventually achieve hemostasis. Applicable in the treatment of acute or chronic, localized or systemic primary fiber fibrinolytic hyperthyroidism caused by bleeding, such as obstetric hemorrhage, renal hemorrhage, hemorrhage of hypertrophy of the prostate, hemophilia, pulmonary tuberculosis hemoptysis, stomach bleeding, after operation of liver, lung, spleen and other viscera hemorrhage; also can be used in surgery when abnormal bleeding etc.. Clinical tranexamic acid has effect significantly to insect bites disease, dermatitis and eczema, simple purpura, chronic urticaria, artificial sex urticaria, toxic eruption and eruption. And also has a certain effect on erythroderma, scleroderma, systemic lupus erythematosus (SLE), Erythema multiforme, shingles and alopecia areata. Treatment of hereditary angioedema effect is also good. In the treatment of Chloasma, general medicine is effective about 3 weeks, markedly effective 5 weeks, a course of 60 days. Given orally in doses of 0.25 ~ 0.5 g, a day 3 ~ 4 times. A few patients can nausea, fatigue, pruritus, abdominal discomfort, and diarrhea side effects after withdrawal symptoms disappear. |
||||||||
| Indications |
|
||||||||
| Mechanism of action | Tranexamic acid is a synthetic lysine amino acid derivative, which diminishes the dissolution of hemostatic fibrin by plasmin. In the presence of tranexamic acid, the lysine receptor binding sites of plasmin for fibrin are occupied, preventing binding to fibrin monomers, thus preserving and stabilizing fibrin’s matrix structure. | ||||||||
| Pharmacokinetics | After a single oral administration of two 650 mg tablets of LYSTEDA, the peak plasma concentration (Cmax ) occurred at approximately 3 hours (Tmax ). The absolute bioavailability of LYSTEDA in women aged 18-49 is approximately 45%. Following multiple oral doses (two 650 mg tablets three times daily). administration of LYSTEDA for 5 days, the mean C max increased by approximately 19% and the mean area under the plasma concentration-time curve (AUC) remained unchanged, compared to a single oral dose administration (two 650 mg tablets). Plasma concentrations reached steady state at the 5th dose of LYSTEDA on Day 2. | ||||||||
| Side effects | The table below contains some of the side-effects associated with tranexamic acid, although these occur only rarely.
|
||||||||
| Chemical Properties | white crystalline powder | ||||||||
| Uses | Antifibrinolytic agent; blocks lysine binding sites of plasminogen. Hemostatic. | ||||||||
| Uses | Used as lysine analogue to characterize binding sites in plasminogen | ||||||||
| Uses | Fibrinolysis, the cleavage of fibrin by plasmin, is a normal step in the dissolution of fibrin clots after wound repair. Tranexamic acid is an inhibitor of fibrinolysis that blocks the interaction of plasmin with fibrin (IC50 = 3.1 μM). It is a lysine mimetic that binds the lysine binding site in plasmin. Antifibrinolytic agents have value when fibrinolytic activity is abnormally high or when coagulation is impaired. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Ami Lifesciences Pvt Ltd
- Tranexamic acid 99%
- Inquiry
- 2024-02-17
-
VIP1年
- PROTECH TELELINKS
- 1197-18-8 98%
- Inquiry
- 2024-02-09
-
VIP1年
- Rivashaa Agrotech Biopharma Pvt. Ltd.
- 1197-18-8 Tranexamic acid 98%
- Inquiry
- 2024-01-25
-
VIP1年
- Shilpa Medicare Limited (SML)
- Tranexamic acid 1197-18-8 99%
- Inquiry
- 2024-01-11
-
VIP1年
- Malladi Drugs AND Pharmaceuticals Limited
- Tranexamic acid 99%
- Inquiry
- 2024-01-09
-
VIP1年
- Venture Pharmaceuticals pvt ltd
- 1197-18-8 98%
- Inquiry
- 2024-01-09
-
VIP1年
- Kawman Pharma (A Division of K P Manish Global Ingredients Pvt Ltd)
- 1197-18-8 Tranexamic acid 98%
- Inquiry
- 2024-01-08
-
VIP1年
- CNS CHEMICALS
- Tranexamic acid 98%
- Inquiry
- 2024-01-03
-
VIP1年
- Medi Pharma Drug House
- Tranexamic Acid 99%
- Inquiry
- 2023-12-28
-
VIP1年
- GGC Chemicals And Pharmaceuticals Pvt Ltd
- 1197-18-8 99%
- Inquiry
- 2023-12-22
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
