| Product Name: |
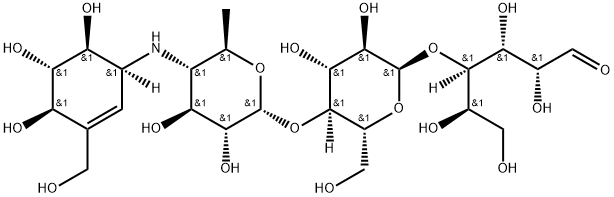
Acarbose |
| Synonyms: |
Acarbose Hydrate;AMylostatin J, Bay g 5421, alpha-GHI;4)-O-a-D-glucopyranosyl-(1®;D-Glucose,O-4,6-dideoxy-4-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxyMethyl)-2-cyclohexen-1-yl]aMino]-a-D-glucopyranosyl-(1®;Acarbose (Acarbosum);(2R,3R,4R,5S,6R)-5-((2R,3R,4R,5S,6R)-5-;O-4,6-Dideoxy-4-[[[1S-(1a,4a,5b,6a)]-4,5,6-trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino]-a-D-glucopyranosyl-(1-4)-O-a-D-glucopyranosyl-(1-4)-D-gluco-�se;Prandase |
| CAS: |
56180-94-0 |
| MF: |
C25H43NO18 |
| MW: |
645.6 |
| EINECS: |
260-030-7 |
| Product Categories: |
API;SEROQUEL;Sugars, Carbohydrates & Glucosides;Intermediates & Fine Chemicals;Oligosaccharides;Pharmaceuticals;API's |
| Mol File: |
56180-94-0.mol |
 |
| |
| Acarbose Chemical Properties |
| Melting point |
165-170°C |
| alpha |
D18 +165° (c = 0.4 in water) |
| Boiling point |
675.05°C (rough estimate) |
| density |
1.4278 (rough estimate) |
| refractive index |
1.6000 (estimate) |
| storage temp. |
Store at RT |
| Water Solubility |
Soluble in water. |
| Merck |
14,18 |
| InChIKey |
XUFXOAAUWZOOIT-JMPDRRIHSA-N |
| Safety Statements |
24/25 |
| RTECS |
LZ7153000 |
| HS Code |
29400090 |
| |
| Acarbose Usage And Synthesis |
| Overview |
Acarbose is an alpha-glucosidase inhibitor drug that is used in the treatment of type 2 diabetes mellitus. It is normally given to patients who are non-insulin dependent where oral hypoglycemic agents or diet modification do not control their condition. The drug is commonly sold in China and Europe as Glocobay while in North America it is sold using the Precose brand name. Acarbose is an oligosaccharide that is normally formed by the process of fermenting a microorganism known as Actinoplanes utahensis. The drug is white and is soluble in water. |
| Pharmacology |
Acarbose acts by delaying the digestion of consumed carbohydrates, leading to a small increase in blood sugar levels. Acarbose minimizes the concentrations of glycosylated hemoglobin in patients with type 2 mellitus. |
| Mechanism of Action |
Acarbose does not enhance the secretion of insulin, which is in contrast to sulfonylureas. The drug’s antihyperglycemic action is because of competitive, reversible inhibition of pancreatic alpha-amylase and intestinal alpha-glucoside hydrolase enzymes that are membrane-bound. On one hand, the membrane-bound intestinal alpha-glucosidases hydrolyze trisaccharides, disaccharides, and oligosaccharides to glucose as well as other monosaccharides in the small intestine’s brush border. Through this enzyme inhibition in diabetic patients, postprandial hyperglycemia is lowered and glucose absorption is delayed. |
| Information on Dosing |
Acarbose acts by preventing the digestion of complex carbohydrates; therefore, it should be administered orally at the start (with the first bite) of every meal, three times a day. It is important to note that patients should adhere to dietary instructions, regular testing of blood and urine glucose levels as well as regular exercise. However, Acarbose does not lead to hypoglycemia even when patients take it in a fasted state. Conversely, Acarbose is administered in combination with sulfonylurea drugs and insulin, thus can lead to low blood sugar, increasing the hypoglycemic potential of these agents.
It is noteworthy that Acarbose dosage should always be individualized based on both tolerance and effectiveness but not exceeding the maximum recommended dose of 100mg.t.i.d depending on the patient’s weight. In addition, if the prescribed diet is not adhered to, side effects in the intestines might increase. During the initiation of treatment, therapeutic response to Acarbose can be determined by one-hour postprandial plasma glucose as well as identifying the maximum effective dose for a specific patient. Glycosylated hemoglobin should then be measured at intervals of about three months,
The main aim of using Acarbose is to reduce glycosylated hemoglobin and postprandial plasma glucose levels to near normal by administering the lowest effective dose. |
| Acarbose Warnings |
Before administering Acarbose to a patient, the doctor must ensure that the patient has ever had cirrhosis of the liver or any other liver disease, an inflammatory disease, or ketoacidosis. Acarbose is contraindicated in individuals that are known to exhibit hypersensitivity to the drug. In particular, patients with diabetic cirrhosis or ketoacidosis. The drug is also contraindicated in people with colonic ulceration, inflammatory bowel disease, and partial intestinal obstruction. Furthermore, Acarbose is contraindicated in individuals with intestinal disorders as well as conditions that may deteriorate due to increased levels of gas in the intestine.
Patients should inform the doctor or health worker that they are on Acarbose medication before having any surgery. Pregnant women should not also take the drug unless prescribed by a doctor. |
| Side effects |
Side effects normally develop during the first weeks of using the drug. Notably, they are primarily mild-to-moderate gastrointestinal effects.
One of the major side effects of Acarbose is the delivery of the remaining carbohydrate after degradation of some into glucose to the colon. Studies have revealed that 78% who use Acarbose experience flatulence and diarrhea as a result of digestion of the complex carbohydrates in the colon.
Most Acarbose effects are dose-related, therefore, it is advisable to begin with small dosage first and gradually increasing to the required amount of dose. The initial dosage is 25 mg t.i.d, which commonly given three times a day. After 4-8 weeks intervals, the maintenance dosage can be increased to 50mg t.i.d. The maximum dosage is 50mg t.i.d for patients who weigh less than 60kg while for those weighing above 60kg, it should be a maximum of 100 mg t.i.d. it is noteworthy that studies have indicated that gastrointestinal side effects can decrease from 50% to 15% over the intervals or even at the maintenance dosing stage.
A doctor or physician should be immediately called if a patient suffers from hyperglycemia. The patient can also eat food comprising of monosaccharides such as gel or glucose tablet.
The doctor should be informed immediately if a patient is experiencing fevers, unusual stress, and illness when taking Acarbose since such events typically lead to a change of blood sugar levels thus affecting the required dosage.
Studies have revealed that Acarbose can help patients with diabetes to lose weight.
Severe Acarbose side effects include severe itching, constipation, unusual bleeding, and yellowing of eyes and skin. |
| Chemical Properties |
Off-White Solid |
| Uses |
antipsychotic: 5HT antagonist, dopamine antagonist, H1-antihistamine, alpha adrenergic blocker |
| Uses |
Acarbose is pseudo-oligosaccharide with a terminal C7-cyclitol patented in 1975 by Bayer. Acarbose is a component of the amylostatin complex produced by a species of Actinoplanes and Streptomyces. Acarbose acts as a potent inhibitor of alpha-glucosidases and saccharases. Since 1990, acarbose has been used therapeutically for the treatment of type 2 diabetes. |
| Definition |
ChEBI: A tetrasaccharide derivative consisting of a dideoxy-4-{[4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl C7 cyclitol moiety [called valienol (or valienamine)] linked via nitrogen to isomaltotriose. |
|



