


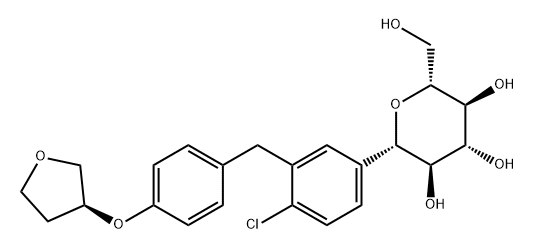
Empagliflozin
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameEmpagliflozin
- CAS No.864070-44-0
- EINECS No.620-176-8
- MFC23H27ClO7
- MW450.91
- AppearancesolidWhite
- density 1.398
- Boiling point 665℃
- storage temp. 2-8°C
AD68
| Empagliflozin Basic information |
| Product Name: | Empagliflozin |
| Synonyms: | EMpagliflozin;BI-10773;(1S)-1,5-Anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-D-glucitol;EMpagliflozin/ BI-10773;EMPAGLIFLBZIN;(2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-((S)-tetrahydrofuran-3-yloxy)benzyl)phenyl)-6-(hydroxyMethyl)-tetrahydro-2H-pyran-3,4,5-triol;D-Glucitol, 1,5-anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]M ethyl]phenyl]-, (1S)-;(1S)-1,5-Anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-D-glucitol Empagliflozin BI 10773 |
| CAS: | 864070-44-0 |
| MF: | C23H27ClO7 |
| MW: | 450.90928 |
| EINECS: | 1592732-453-0 |
| Product Categories: | API;Inhibitors;Empagliflozin |
| Mol File: | 864070-44-0.mol |
 |
|
| Empagliflozin Chemical Properties |
| Boiling point | 665℃ |
| density | 1.398 |
| Fp | 356℃ |
| Safety Information |
| Empagliflozin Usage And Synthesis |
| Description | Empagliflozin(trade name Jardiance) is an inhibitor of the sodium glucose co-transporter-2 (SGLT-2), and causes sugar in the blood to be excreted by the kidneys and eliminated in urine. On August 1, 2014, the Food and Drug Administration (FDA) officially approved the drug for the treatment of type 2 diabetes, to improve and control blood glucose of adults. Empagliflozin is the third SGLT-2 inhibiting drugs approved by FDA. Another two SGLT-2 inhibitor drugs, canagliflozin and dapagliflozin, belonging to Johnson Pharmaceuticals, AstraZeneca and Bristol-Myers Squibb respectively, are approved by FDA in November 2013 and January 2014 respectively. The new drug Empagliflozin’s application to FDA can be described as twists and turns. In March 2014, due to a large particle contamination incident at the empagliflozin production plant in Boehringer, the application of new drug submitted by the Boehringer-Eli Lilly Alliance was rejected by FDA. In June 2014, after reviewing the summary and material submitted by Boehring in March, confirming that the quality management and compliance system of the Boehring drug production facility was acceptable, FDA withdrew the previously issued warning letter. The Boehringer-Eli Lilly Alliance also filed an application with the FDA on June 17th. |
| Clinical research | FDA approval of empagliflozin(Jardiance) was based on the results of seven clinical trials conducted in nearly 4,500 patients with type 2 diabetes mellitus. Compared to the placebo group, all patients taking empagliflozin(Jardiance) had a significantly lower HbA1c level. The most common adverse reactions are urinary tract infections and female reproductive system infections. On March 21, 2014, European Medicines Agency (EMA) Commission on Human Medicines recommended the approval of the sodium glucose co-transporter-2 (SGLT2) inhibitor (empagliflozin) for the treatment of adult type 2 diabetes mellitus. On May 23, 2014, European Medicines Agency (EMA) approved for empagliflozin’s listing in Europe. On June 16, 2014, the Boehringer-Erya Diabetes Federation released the latest data of empagliflozin Phase II clinical trials at the 74th American Diabetes Association Science Conference (ADA2014). In a two-year study, empagliflozin or glimepiride was used in the treatment of adult patients with type 2 diabetes based on metformin. The results showed that empagliflozin(Jardiance) reduced HbA1c more significantly compared with glimepiride, while the effect of weight and blood pressure treatment are equivalent with glimepiride. Another 52-week study was conducted in patients with type 2 diabetes who were still unable to adequately control blood glucose levels with high doses of insulin (with or without metformin). The results showed that compared with placebo, Jardiance significantly reduced blood glucose levels and weight, and reduced the dose of insulin, with Jardiance or placebo on a daily basis with multiple injections of insulin. In both studies, the safety of empagliflozin was consistent with previous studies. FDA-related reports write that the drug should not be used in the following types of patients: type 1 diabetes, elevated blood or urine ketones (diabetic ketoacidosis), severe kidney damage, end-stage renal disease or dialysis patients. The most common side effects are urinary tract infections and female genital infections. Empagliflozin can cause dehydration and lead to decreased blood pressure, resulting in patients with dizziness or fainting and decreased renal function. The risk of older patients, especially those with impaired renal function and diuretics, appears to be greater. FDA requires Boehring and Eli Lilly to conduct four post-marketing studies: complete ongoing cardiovascular outcome trials, pediatric pharmacokinetics and pharmacodynamics trials, pediatric safety and efficacy trials, toxicity test for the drug (especially effect for renal function, bone and growth and development) |
| Precautions | Do NOT use empagliflozin if:
|
| References | https://en.wikipedia.org/wiki/Empagliflozin https://www.drugs.com/cdi/empagliflozin.html |
| Uses | Empagliflozin is a novel, potent and selective SGLT-2 inhibitor, improves glycaemic control and features of metabolic syndrome in diabetic rats. |
| Definition | ChEBI: A C-glycosyl compound consisting of a beta-glucosyl residue having a (4-chloro-3-{4-[(3S)-tetrahydrofuran-3-yloxy]benzyl}phenyl group at the anomeric centre. A sodium-glucose co-transporter 2 inhibitor u ed as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Aptra Synthesis Pvt Ltd
- Empagliflozin 864070-44-0 99%
- Inquiry
- 2024-05-20
-
VIP1年
- Aspen Biopharma Labs Pvt Ltd
- 864070-44-0 Empagliflozin 98%
- Inquiry
- 2024-03-16
-
VIP1年
- Sibram Pharmaceutical
- 864070-44-0 99%
- Inquiry
- 2024-03-16
-
VIP1年
- Vijaya Pharma And Life Science
- Empagliflozin 98%
- Inquiry
- 2024-03-13
-
VIP1年
- Century Pharmaceuticals Ltd
- 864070-44-0 Empagliflozin 99%
- Inquiry
- 2024-03-07
-
VIP1年
- Mylan Laboratories Ltd
- Empagliflozin 864070-44-0 98%
- Inquiry
- 2024-03-06
-
VIP1年
- Basis Laboratories Private Limited
- Empagliflozin 98%
- Inquiry
- 2024-03-05
-
VIP1年
- Rini Life Science Pvt Ltd
- 864070-44-0 99%
- Inquiry
- 2024-03-02
-
VIP1年
- Cohance Lifesciences (Previously RA Chem Pharma Ltd)
- 864070-44-0 Empagliflozin 98%
- Inquiry
- 2024-02-28
-
VIP1年
- Exemed Pharmaceuticals (Oneiro Chemicals Pvt Ltd)
- Empagliflozin 864070-44-0 98%
- Inquiry
- 2024-02-28
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
