

Boscalid
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:1000KG
- Time:2019-07-06
Product Details
- Product NameBoscalid
- CAS No.188425-85-6
- EINECS No.203-625-9
- MFC18H12Cl2N2O
- MW343.21
- Appearanceneat &_& solidWhite to Almost white
- storage temp. Inert atmosphere,2-8°C
- Melting point 142.8-143.8°.
- density 1.381
- Boiling point 447.7±45.0 °C(Predicted)
- Water Solubility Practically insoluble in water
JD607
| Product Name: | Boscalid |
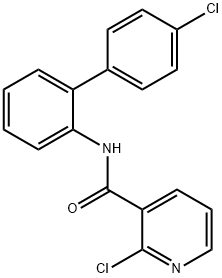
| Synonyms: | BOSCALID;2-CHLORO-N-(4'-CHLORO-2-BIPHENYLYL)NICOTINAMIDE;2-CHLORO-N-(4'-CHLOROBIPHENYL-2-YL)-NICOTINAMIDE;2-CHLORO-N-[2-(4-CHLOROPHENYL)PHENYL]-PYRIDINE-3-CARBOXAMIDE;NICOBIFEN;boscalid solution;2-Chloro-N-(4μ-chloro-2-biphenylyl)nicotinamide, Nicobifen;Boscalid 100mg [188425-85-6] |
| CAS: | 188425-85-6 |
| MF: | C18H12Cl2N2O |
| MW: | 343.21 |
| EINECS: | 203-625-9 |
| Product Categories: | |
| Mol File: | 188425-85-6.mol |
 |
|
| Boscalid Chemical Properties |
| Melting point | 142.8-143.8°. |
| density | 1.381 |
| Fp | 4 °C |
| storage temp. | 0-6°C |
| Merck | 14,1352 |
| EPA Substance Registry System | 3-Pyridinecarboxamide, 2-chloro-N-(4'-chloro[ 1,1'-biphenyl]-2-yl)- (188425-85-6) |
| Safety Information |
| Hazard Codes | F,Xn,N |
| Risk Statements | 11-38-48/20-63-65-67-51/53 |
| Safety Statements | 36/37-62-61 |
| RIDADR | UN1294 3/PG 2 |
| RTECS | US4587550 |
| HazardClass | 9 |
| PackingGroup | III |
| Hazardous Substances Data | 188425-85-6(Hazardous Substances Data) |
| Boscalid Usage And Synthesis |
| Fungicide | Boscalid is a kind of nicotinamide germicide first successfully developed by BASF of Germany. It has a broad spectrum of bactericidal activity and has a preventive effect, being active against almost all types of fungal diseases. It has excellent effects on the control of powdery mildew, gray mold, root rot disease, sclerotinia and various kinds of rot diseases and is not easy to produce cross-resistance. It is also effective against the resistant bacteria to other agents. It is mainly used for the prevention and control of diseases associated with rape, grapes, fruit trees, vegetables and field crops. The results have showed that Boscalid had a significant effect on the treatment of Sclerotinia sclerotiorum with both the disease incidence control effect and the disease control index being higher than 80%, which was better than any of the other agents currently popularized. It has a significantly higher control efficacy than carbendazim. Apply 50% boscalid water solution dispersible granules to control rape sclerotia disease with a dose of 24 to 36 grams per acre medication per general year. In severe years, apply 36 to 48 grams per acre for medication. |
| Toxicity | (1) Mammalian toxicity: rat acute oral LD50> 5,000 mg/kg; Rat acute pertacuneous LD50 > 2,000 mg/kg, being non-irritating to skin and eyes of rabbits without sensitization to guinea pig skin. Rats inhaled LC50 (4 h)> 6.7 mg/L. NOEL: Rats, approximately 5 mg/kg (b. w.); Chronic NOAEL: 21.8 mg/kg (b. w.). [2003]; ADI/RfD (JMPR) 0.04 mg/kg (b.w.) [2006]; (EC) 0.04 mg/kg (b.w.) [2008]; cRfD 0.218 mg/kg (b.w.) [2003], (FSC) 0.044 mg/kg (b.w.) [2006]; other: no mutagenicity (Ames test, mouse), teratogenicity (rat, rabbit) and carcinogenic effects (dog, rat, mice); no adverse effects on reproduction (rat). (2) Ecotoxicity: Birds: Quail LD50> 2,000 mg/kg (b.w.). Fish: Rainbow trout LC50 (96 h) was 2.7 mg/L. Daphnia: EC50 (48 h) was 5.33 mg/L. The algae: Pseudokirchneriella subcapitata ErC50 (96 h) was 3.75 mg/L. Other aquatic organisms: Chironomus riparius NOEC 2.0 mg/L. Bee: NOEC (oral) is 166 μg /bee, (contact) is 200 μg/bee. Earthworm: Eisenia foetida LC50> 1,000 mg/kg (dry soil). (3) Environmental toxicity: animal: biphenyl ring is first subject to hydroxylation, followed by glucosylation and sulfation reaction. Boscalid is rapidly and extensively subject to metabolism in the body and excreted rapidly through the feces. Plants: biphenyl and pyridine ring are subject to hydroxylation and further ring-opening reaction. However, the parent compound, which has not been structurally altered, remains to be a major part of the residue. Soil/environment: it has moderate degradation action in the soil with the soil DT50 being 108 d ~> 1 a (laboratory, aerobic conditions, 20 ℃); the DT50 of the field is about 28d~200d. It has a excellent degradation property in natural water/sediment systems. |
| Mechanism | Boscalid is a kind of mitochondrion respiration inhibitor, being the inhibitor of the succinate dehydrogenase (SDHI) that acts by inhibiting succinate coenzyme Q reductase (also known as complex II) on the mitochondrial electron transport chain, with its mechanism of action being similar as that of other kinds of amide and benzamide fungicides. It has effects on the entire growth period of the pathogen, especially having a strong inhibitory effect against the spore germination. It also has excellent prophylactic effects and excellent intra-leaf permeability. Boscalid is a foliar application germicide, which can penetrate vertically and be transmitted to the top of the plant leaves. It has excellent preventive effect and has certain therapeutic effect. It can also inhibit the spore germination, germ tube elongation and attachment formation, and is effective in all other growth stages of the fungus, exhibiting excellent resistance to rain erosion and persistence. |
| Uses | Boscalid is a broad-spectrum, systemic fungicide, which can effectively control the diseases that are resistant to sterol inhibitors, bisimides, benzimidazoles, benzopyrimidines, phenyl amides and methoxy acrylic esters germicide. This product can be transported to the top of the plant through the xylem to the tip and leaf margin; it also has a vertical penetration effect, being able to be transmitted through the leaf tissue, to the back of the leaf; however, the product has a very small redistribution action in the vapor phase. Boscalid is mainly through spraying through stem and leaf at a dosage of 100~1,200 g a.i. /hm2. Boscalid can be used for the prevention and treatment of the powdery mildew (Monilinia spp), leaf spot (Mycosphaerella spp) as well as diseases caused by Alternaria spp., Alternaria spp., Botrytis cinerea, Botrytis cinerea, (Botrytis spp), Sclerotinia sclerotiorum (Sclerotinia spp) that have been found in grape, lawn, fruit trees, and ornamental plant. It can also be used in complex formulations for grain, grapes, peanuts and potatoes and other tillage crops. Boscalid also owns single-dosage product such as Cantus, to be used in pear, grape and post-harvest kiwifruit to control gray mold with the usage amount of 1~1.2kg/hm2. It can be used in different growth stages of grape with however, spraying before the grapes form cluster yielding the best efficacy. It also has a wide range of complex products. For example, Bellis (25.2% piperacillin + 12.8% pyrizole) is a broad-spectrum fungicide, being able to be registered for watermelon and pear. The recommendation dosage for its water dispersible granules is 0.6-1.6 kg/hm2 for the control of many diseases, including anthrax, Botrytis cinerea, Monilinia spp and black spot disease. This product has been developed in Latin America and Italy. Another example is that Signum (26.7% piperacillin + 6.7% pyrazole) has been registered for apricot, horticultural products and lettuce for the control of black spot, gray mold and sclerotinia. This product is also water dispersible granules with prevention effect. Furthermore, the germicide for fruit trees, Naria (Pyraclostrobin + boscalid) has been approved for registration in Japan in 2006 and listed in 2007. Naria is widely used in the control of many diseases in apple, Japanese pear, cherry and peach. It is especially suitable for the prevention of some refractory diseases in summer such as: leaf spot, black spot, scab, anthrax, ring rot and powdery mildew. |
| Preparation | 1. Take o-chloronitrobenzene as raw material, first have it react with chlorobenzene boronic acid to undergo Suziki reaction, followed by reduction, and finally condensation with 2-chloronicotinyl chloride to obtain Boscalid crude product. 2. Take o-iodine aniline as raw material, first have reaction with 2-chloronicotinyl chloride, followed by Suzuki reaction with chlorobenzene boric acid to obtain the finished product. |
| Patents | On November 7, 2012, the patent on the European patent expired. On November 9, 2012, its patents in the United States expire. November 9, 2012, the administrative protection period of the compound in China expires. In 2003, Boscalid was registered in the United States and obtained a 10-year registration of data protection. In August 1, 2008, Boscalid was listed in the Appendix 1 of the EU pesticide registration directive (91/414) with its registration information being protected to until July 31, 2018. The patent and administrative protection in Europe and the United States and China will soon expire, but the EU data protein is far lagged behind from its patent protection. Non-patented products manufacturers, if needs to enter the European and American markets, must prepare a complete set of registration information, or consult the data owner. Alternatively, they can enter into the markets until the information protein is expired. However, boscalid will soon become a focus of those non-patented manufactures in China, which will lead to the escalation of the Chinese market completion of boscalid. |
| Chemical Properties | Light Beige Solid |
| Uses | Boscalid is a fungicide belonging to the class of carboxamides. Boscalid acts by inhibiting spore germination, germ tube elongation and is also effective on all other stages of fungal development. Bos calid is used in the agriculture to protect crops from gray mold, powdery mildew and other fungus. |
| Definition | ChEBI: A pyridinecarboxamide obtained by formal condensation of the carboxy group of 2-chloronicotinic acid with the amino group of 4'-chlorobiphenyl-2-amine. A fungicide active against a broad range of fungal pathogens including Botrytis spp., |
| Uses | Fungicide. |
| Toxicity | LD50 in rats (mg/kg): >5000 orally; >2000 dermally (U.S. EPA) |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
