

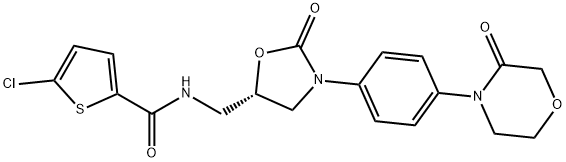
Rivaroxaban
| Price | USD100.00 |
| Packge | 25KG |
- Min. Order:1KG
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameRivaroxaban
- CAS No.366789-02-8
- EINECS No.1308068-626-2
- MFC19H18ClN3O5S
- MW435.88
- AppearancesolidWhite to off-white
- Melting point 228-229°C
- storage temp. Inert atmosphere,2-8°C
- Boiling point 732.6±60.0 °C(Predicted)
- density 1.460±0.06 g/cm3(Predicted)
CR075
| Rivaroxaban Basic information |
| Product Name: | Rivaroxaban |
| Synonyms: | (S)-Rivaroxaban;(S)-5-Chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)-oxazolidin-5-yl)methyl)thiophene-2-carbox;RIVAROXABAN STAGE-IV [5-CHLORO-N-({5S)-2-OXO-3-[4-(3-OXO-4-MORPHOLINYL)PHENYL]-1,3-OXAZOLIDIN-5-YL}-METHYL)-2(AS PER INV;Rivaroxaban Isomer;5-Chloro-N-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]-2-thiophenecarboxamide;BAY 59-7939;Rivaroxaban;5-Chloro-N-(((5S)-2-oxo-3-(4-(3-oxomorpholin-4-yl)phenyl)-1,3-oxazolidin-5-yl)methyl)thiophene-2-carboxamide |
| CAS: | 366789-02-8 |
| MF: | C19H18ClN3O5S |
| MW: | 435.88 |
| EINECS: | 1308068-626-2 |
| Product Categories: | Chiral Reagents;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds;API;Inhibitor;Rivaroxaban;Pharmaceutical raw materials;Inhibitors |
| Mol File: | 366789-02-8.mol |
 |
|
| Rivaroxaban Chemical Properties |
| Melting point | 228-229°C |
| storage temp. | Refrigerator |
| Safety Information |
| Rivaroxaban Usage And Synthesis |
| Description | Rivaroxaban is a pure (S)-enantiomer. It is an odorless, non-hygroscopic, white to yellowish powder. Rivaroxaban is only slightly soluble in organic solvents (e.g., acetone, polyethylene glycol 400) and is practically insoluble in water and aqueous media. Rivaroxaban, a FXa inhibitor, is the active ingredient in XARELTO Tablets. XARELTO a kind of orally anticoagulant drug which is used for the prevention of blood clots. Rivaroxaban takes effect through competitively inhibiting free and clot bound factor Xa, which is needed to activate prothrombin (factor II) to thrombin (factor IIa). The later is a serine protease that is required to activate fibrinogen to fibrin, which is the loose meshwork that completes the clotting process.  Each XARELTO tablet contains 10 mg, 15 mg, or 20 mg of rivaroxaban. The inactive ingredients of XARELTO are: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. Additionally, the proprietary film coating mixture used for XARELTO 10 mg tablets is Opadry(R) Pink and for XARELTO 15 mg tablets is Opadry(R) Red, both containing ferric oxide red, hypromellose, polyethylene glycol 3350, and titanium dioxide, and for XARELTO 20 mg tablets is Opadry(R) II Dark Red, containing ferric oxide red, polyethylene glycol 3350, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide. |
| Indications and Usage | Rivaroxaban is an antithrombotic drug and was developed in a collaboration between the German Bayer Pharmaceuticals and American Johnson company. It is different from the traditional antithrombotic drug heparin in that Rivaroxaban does not need the participation of antithrombin III and can directly antagonize free and bound Xa factors. Heparin requires the effects of antithrombin III, and has no effect on Xa factors in the prothrombin complex. Rivaroxaban is the first oral direct Xa factor inhibitor in the whole world, and it can selectively and competitively inhibit free and bound Da factors as well as prothrombin activity. It uses a dose-dependent method to extend the partial thromboplastin time (PTT) and activated partial thromboplastin time (aPTT) to extend clotting time and lower thrombin genesis. Rivaroxaban has the characteristics of high bioavailability, wide range of target diseases, stable dose-effect relationship, easy oral intake, and low bleeding risk. Rivaroxaban can also prevent and treat venous thrombosis. It is mainly used clinically to prevent deep venous thrombosis (DVT) and pulmonary embolism (PE) in adult patients following hip and knee replacement surgery. It is also used to prevent patients with nonvalvular atrial fibrillation from cerebral apoplexy and noncentral nervous system embolism, and it can lower the recurrence risk of coronary syndromes. |
| Mechanism of Action | Rivaroxaban is a selective inhibitor of FXa. It does not require a cofactor (such as Anti-thrombin III) for activity. Rivaroxaban inhibits free FXa and prothrombinase activity. Rivaroxaban has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa, rivaroxaban decreases thrombin generation. |
| What is XARELTO? | XARELTO (Rivaroxaban) is a prescription medicine used to:
|
| Uses | Rivaroxaban is recommended as an option for treating pulmonary embolism and preventing recurrent deep vein thrombosis and pulmonary embolism in adults.
|
| important information about XARELTO | For people taking XARELTO for atrial fibrillation: People with atrial fibrillation (an irregular heart beat) are at an increased risk of forming a blood clot in the heart, which can travel to the brain, causing a stroke, or to other parts of the body. XARELTO lowers your chance of having a stroke by helping to prevent clots from forming. If you stop taking XARELTO, you may have increased risk of forming a clot in your blood. XARELTO can cause bleeding which can be serious, and rarely may lead to death. This is because XARELTO is a blood thinner medicine that reduces blood clotting. While you take XARELTO you are likely to bruise more easily and it may take longer for bleeding to stop. You may have a higher risk of bleeding if you take XARELTO and take other medicines that increase your risk of bleeding, including:
|
| Carcinogenesis, Mutagenesis, Impairment of Fertility | Rivaroxaban was not carcinogenic when administered by oral gavage to mice or rats for up to 2 years. The systemic exposures (AUCs) of unbound rivaroxaban in male and female mice at the highest dose tested (60 mg/kg/day) were 1- and 2-times, respectively, the human exposure of unbound drug at the human dose of 20 mg/day. Systemic exposures of unbound drug in male and female rats at the highest dose tested (60 mg/kg/day) were 2- and 4-times, respectively, the human exposure. Rivaroxaban was not mutagenic in bacteria (Ames-Test) or clastogenic in V79 Chinese hamster lung cells in vitro or in the mouse micronucleus test in vivo. No impairment of fertility was observed in male or female rats when given up to 200 mg/kg/day of rivaroxaban orally. This dose resulted in exposure levels, based on the unbound AUC, at least 13 times the exposure in humans given 20 mg rivaroxaban daily. |
| Adverse Reactions | Bleeding events (may be serious or fatal), back pain, wound secretion, pruritus, pain in extremity, abdominal pain, blister. |
| Chemical Properties | White Solid |
| Uses | anti-coagulant; Factor X inhibitor,Rivaroxaban is an orally active, direct inhibitor of Factor Xa (Ki = 0.4 nM), which is a crucial component of the intrinsic and extrinsic pathways of the blood coagulation cascade.It demonstrates >10,000-fold greater selectivity for Factor Xa compared to other related serine proteases.In various animal arterial and venous thrombosis models, rivaroxaban is reported to inhibit thrombin formation without prolonging bleeding time and has been approved for clinical use as an anticoagulant in the prevention of stroke and the treatment of venous thromboembolisms. |
| Uses | A novel antithrombotic agent. A highly potent and selective, direct FXa inhibitor |
| Definition | ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of 5-chlorothiophene-2-carboxylic acid with the amino group of 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholin-3-one. An anticoagulant u ed for prophylaxis of venous thromboembolism in patients with knee or hip replacement surgery. |
| Rivaroxaban Preparation Products And Raw materials |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Scimplify
- Rivaroxaban-IHS/IP 366789-02-8 99%
- Inquiry
- 2024-10-25
-
VIP1年
- SETV ASRV LLP
- 366789-02-8 95-99 %
- Inquiry
- 2024-09-11
-
VIP1年
- AKASH PHARMA EXPORTS
- Rivaroxaban 99%
- Inquiry
- 2024-05-16
-
VIP1年
- ADAARSH PHARMA
- Rivaroxaban-IHS/IP 99%
- Inquiry
- 2024-05-03
-
VIP1年
- Chon Chem Industries
- 366789-02-8 Rivaroxaban 98%
- Inquiry
- 2024-03-30
-
VIP1年
- Omshri Labs Pvt Ltd
- 366789-02-8 Rivaroxaban 99%
- Inquiry
- 2024-03-26
-
VIP1年
- Symed Laboratories Ltd
- Rivaroxaban 98%
- Inquiry
- 2024-03-21
-
VIP1年
- Actis Generics Pvt Ltd
- Rivaroxaban 366789-02-8 98%
- Inquiry
- 2024-03-20
-
VIP1年
- Apionex Pharma Pvt Ltd
- 366789-02-8 98%
- Inquiry
- 2024-03-20
-
VIP1年
- Amoli Organics Pvt Ltd
- 366789-02-8 98%
- Inquiry
- 2024-03-20
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
