99129-21-2
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Clear amber liquid |
|---|---|
| Boiling Point | Decomposes below boiling point |
| Flash Point | 162 °F (72 °C) (Closed cup) /Select Max Herbicide/ |
| Solubility | Soluble in most organic solvents |
| Stability/Shelf Life | Unstable at extreme pH's, temperature and upon exposure to UV light. /Select Max Herbicide/ |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
COMPUTED DESCRIPTORS
| Molecular Weight | 359.9 g/mol |
|---|---|
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 9 |
| Exact Mass | 359.1321926 g/mol |
| Monoisotopic Mass | 359.1321926 g/mol |
| Topological Polar Surface Area | 84.2 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 488 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
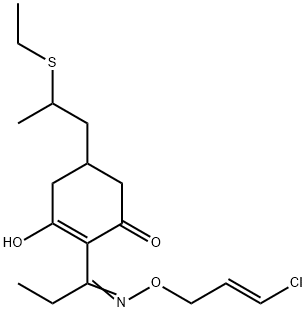
Clethodim is an oxime O-ether resulting from the formal conversion ot the acyclic keto group of 5-[2-(ethylsulfanyl)propyl]-3-hydroxy-2-propionylcyclohex-2-en-1-one to the corresponding oxime with subsequent O-alkylation of the oxime by an (E)-3-chloroallyl group. It is used as a selective postemergence herbicide for the control of annual and perennial grasses in numerous crops, including alfalfa, celery, clover, conifers, cotton, cranberries, garlic, onions, ornamentals, peanuts, soybeans, strawberries, sugarbeet, sunflowers, and vegetables; the (-)-enantiomer has been reported to be more active than the (+)-enantiomer. It has a role as a herbicide and an EC 6.4.1.2 (acetyl-CoA carboxylase) inhibitor. It is an organic sulfide, a cyclic ketone, an organochlorine compound, an oxime O-ether, a beta-diketone and an enol.