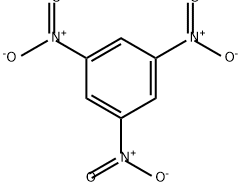
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Trinitrobenzene, wetted with not less than 30% water appears as a light yellow crystalline sludge or slurry. Burns but may require some effort to ignite. A high explosive when dry. Easily ignited and burns very vigorously when dry. Insoluble in water. Produces toxic oxides of nitrogen during combustion. |
|---|---|
| Color/Form | Yellow crystals |
| Odor | Odorless |
| Taste | Tasteless |
| Boiling Point | 315 °C |
| Melting Point | 121.3 °C |
| Solubility | Solubility in water, 278 mg/L at 15 °C |
| Density | 1.688 at 20 °C/4 °C |
| Vapor Pressure | 0.0000032 [mmHg] |
| LogP | log Kow = 1.18 |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitrogen oxides/ and explodes. |
| Heat of Combustion | -663.7 kg cal/g mol wt at 20 °C (solid) |
| Refractive Index | Index of refraction: 1,4775 at 152 C |
| Kovats Retention Index | 284.47 285.91 287.18 |
| Other Experimental Properties | Can be sublimed by careful heating; it is dimorphous, the other (rare) form melts at 61 °C |
| Chemical Classes | Nitrogen Compounds -> Nitros, Aromatic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H228:Flammable solids H301:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P281:Use personal protective equipment as required. P311:Call a POISON CENTER or doctor/physician. P314:Get medical advice/attention if you feel unwell. P391:Collect spillage. Hazardous to the aquatic environment P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 213.10 g/mol |
|---|---|
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 213.00218482 g/mol |
| Monoisotopic Mass | 213.00218482 g/mol |
| Topological Polar Surface Area | 138 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 229 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Trinitrobenzene, wetted with not less than 30% water appears as a light yellow crystalline sludge or slurry. Burns but may require some effort to ignite. A high explosive when dry. Easily ignited and burns very vigorously when dry. Insoluble in water. Produces toxic oxides of nitrogen during combustion.