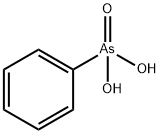
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Benzenearsonic acid is a colorless solid. Used as an analytical reagent for tin. (EPA, 1998) |
|---|---|
| Color/Form | Crystal powder |
| Melting Point | 320 °F decomposes (EPA, 1998) |
| Solubility | Sol in 40 parts water, 50 parts alcohol; insol in chloroform |
| Density | 1.76 (EPA, 1998) - Denser than water; will sink |
| Vapor Pressure | 0.00000111 [mmHg] |
| LogP | log Kow= 0.06 |
| Decomposition | When heated to decomposition it emits toxic fumes of /arsenic/. |
| Dissociation Constants | pKa= 8.48 in water at 25 °C |
| Chemical Classes | Metals -> Arsenic Compounds, Organic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H331:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 202.04 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 201.961114 g/mol |
| Monoisotopic Mass | 201.961114 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 145 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Benzenearsonic acid is a colorless solid. Used as an analytical reagent for tin. (EPA, 1998)