79-06-1
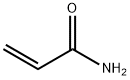
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Acrylamide appears as white crystalline solid shipped either as a solid or in solution. A confirmed carcinogen. Toxic by skin absorption. Less dense than water and soluble in water. May be toxic by ingestion. Used for sewage and waste treatment, to make dyes, adhesives. The solid is stable at room temperature, but upon melting may violently polymerize. Toxic, irritating to skin, eyes, etc. |
|---|---|
| Color/Form | Flake-like crystals from benzene |
| Odor | Odorless |
| Boiling Point | 189 °F at 2 mmHg (EPA, 1998) |
| Melting Point | 184 °F (EPA, 1998) |
| Flash Point | 280.4 °F (EPA, 1998) |
| Solubility | greater than or equal to 100 mg/mL at 72 °F (NTP, 1992) |
| Density | 1.122 at 86 °F (EPA, 1998) - Denser than water; will sink |
| Vapor Density | 2.45 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 0.007 mmHg at 68 °F (EPA, 1998) |
| LogP | log Kow = -0.67 |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Autoignition Temperature | 464 °F (USCG, 1999) |
| Decomposition | Hazardous decomposition products formed under fire conditions - Carbon oxides, nitrogen oxides (NOx). |
| Viscosity | 2.71 cP at 25 °C /50% aqueous solution/ |
| Heat of Vaporization | 61.5-76.5 kJ/mol at 357-413 deg K |
| pH | pH = 5.0-6.5 /50% aqueous solution/ |
| Ionization Potential | 9.50 eV |
| Polymerization | Polymerizes violently at its melting point. |
| Relative Evaporation Rate | Evaporation at 20 °C is negligible |
| Kovats Retention Index | 1943 |
| Other Experimental Properties | Boiling point: 87 °C at 2 mm Hg; 103 °C at 5 mm Hg; 125 °C at 25 mm Hg; readily polymerizes at melting point or under UV light |
| Chemical Classes | Plastics & Rubber -> Other Monomers |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H340:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 71.08 g/mol |
|---|---|
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 71.037113783 g/mol |
| Monoisotopic Mass | 71.037113783 g/mol |
| Topological Polar Surface Area | 43.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 57.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Acrylamide is a colorless, odorless, crystalline solid that can react violently when melted. When it is heated, sharp fumes may be released.Acrylamide is used to make polyacrylamide, which is mainly used in treating waste water discharge from water treatment plants and industrial processes.In addition, acrylamide and polyacrylamides are used in the production of dyes and organic chemicals, contact lenses, cosmetics and toiletries, permanent-press fabrics, paper and textile production, pulp and paper production, ore processing, sugar refining, and as a chemical grouting agent and soil stabilizer for the construction of tunnels, sewers, wells and reservoirs.Acrylamide is formed in foods that are rich in carbohydrates when they are fried, grilled, or baked.