78111-17-8
Product Name:
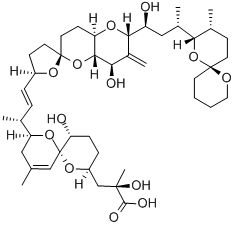
OKADAIC ACID
Formula:
C44H68O13
Synonyms:
OA;Okadaic Acid, Prorocentrum sp. - CAS 78111-17-8 - Calbiochem
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Crystals from dichloromethane/hexane; crystals from benzene-CHCl3 |
|---|---|
| Melting Point | 171-175 °C; 164-166 °C |
| Solubility | Readily soluble in many organic solvents, degrading in acid or base |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Optical Rotation | Specific optical rotation = +21 deg at 20 °C/D (c = 0.33 in CHCl3); +25.4 deg at 25 °C/D (c = 0.24 in CHCl3) |
| Decomposition | Hazardous decomposition products formed under fire conditions. - Carbon oxides |
| Other Experimental Properties | Ionophoric polyether identified in marine organisms |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 805.0 g/mol |
|---|---|
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 10 |
| Exact Mass | 804.46599222 g/mol |
| Monoisotopic Mass | 804.46599222 g/mol |
| Topological Polar Surface Area | 183 Ų |
| Heavy Atom Count | 57 |
| Formal Charge | 0 |
| Complexity | 1520 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 17 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Okadaic acid is a polycyclic ether that is produced by several species of dinoflagellates, and is known to accumulate in both marine sponges and shellfish. A polyketide, polyether derivative of a C38 fatty acid, it is one of the primary causes of diarrhetic shellfish poisoning (DSP). It is a potent inhibitor of specific protein phosphatases and is known to have a variety of negative effects on cells. It has a role as a marine metabolite, an EC 3.1.3.16 (phosphoprotein phosphatase) inhibitor and a calcium ionophore.