78-92-2
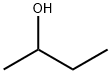
Product Name:
2-Butanol
Formula:
C4H10O
Synonyms:
sec-Butyl alcohol;2-Butanol;sec-Butyl alcohol
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sec-butyl alcohol appears as a clear colorless liquid with an alcohol odor. Flash point below 0 °F. Less dense than water. Vapors heavier than air. Soluble in water. Moderately irritates the eyes and skin. Prolonged and repeated contact may cause defatting and drying of the skin. Vapors may irritate the nose, throat and respiratory tract. May be harmful by ingestion. |
|---|---|
| Color/Form | Colorless liquid |
| Odor | HAS A STRONG VINOUS ODOR |
| Boiling Point | 211 °F at 760 mmHg (NIOSH, 2023) |
| Melting Point | -175 °F (NIOSH, 2023) |
| Flash Point | 75 °F (NIOSH, 2023) |
| Solubility | 16 % (NIOSH, 2023) |
| Density | 0.81 (NIOSH, 2023) - Less dense than water; will float |
| Vapor Density | 2.6 (Air= 1) |
| Vapor Pressure | 12 mmHg (NIOSH, 2023) |
| LogP | log Kow= 0.61 |
| Henry's Law Constant | Henry's Law constant = 9.06X10-6 atm-cu m/mol @ 25 °C |
| Stability/Shelf Life | FEASIBLE AUTO OXIDATION BY ATMOSPHERIC OXYGEN TO FORM PEROXIDES & PEROXY CMPD |
| Autoignition Temperature | 763 °F (406 °C) (IN AIR); 711 °F (377 °C) (IN OXYGEN) |
| Decomposition | When heated to decomposition it emits acrid smoke & fumes. |
| Viscosity | 4.21 cP at 15 °C |
| Corrosivity | sec-Butyl alcohol will attack some forms of plastics, rubber, and coatings. |
| Heat of Combustion | -15,500 BTU/lb= -8600 cal/g= -360x10+5 J/kg |
| Heat of Vaporization | 49.72 kJ/mol @ 25 °C |
| Surface Tension | 23.0 dynes/cm= 0.023 N/m at 20 °C |
| Ionization Potential | 10.10 eV |
| Odor Threshold | Odor Threshold Low: 0.12 [mmHg] Odor Threshold High: 13.8 [mmHg] Detection odor threshold from AIHA (mean = 3.2 ppm) |
| Dielectric Constant | Dielectric constant: 16.6 at 25 °C; dipole moment: 1.66 dehye (in benzene at 30 °C) |
| Refractive Index | Index of refraction: 1.3978 @ 20 °C/D |
| Dissociation Constants | pKa = 17.6 |
| Relative Evaporation Rate | 1.3 (butyl acetate= 1) |
| Kovats Retention Index | 600.6 602.2 565 585 586 565 585 587 594 576 577 605 577 605 577 605 585 586 570 584 586 586 590 593 595 586 586 586 586 591 582 588.4 589 613 587 587.64 561.1 596 585 585 624 591 596 603 586 628 596.4 |
| Other Experimental Properties | IT EXISTS AS D, L, & DL ISOMERS |
| Chemical Classes | Solvents -> Alcohols (<C12) |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 74.12 g/mol |
|---|---|
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 74.073164938 g/mol |
| Monoisotopic Mass | 74.073164938 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 19.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sec-butyl alcohol appears as a clear colorless liquid with an alcohol odor. Flash point below 0 °F. Less dense than water. Vapors heavier than air. Soluble in water. Moderately irritates the eyes and skin. Prolonged and repeated contact may cause defatting and drying of the skin. Vapors may irritate the nose, throat and respiratory tract. May be harmful by ingestion.