7789-59-5
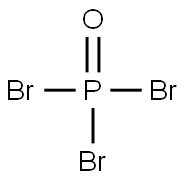
Product Name:
Phosphorus oxybromide
Formula:
Br3OP
Synonyms:
Phosphorus oxide bromide;Phosphorus oxide tribromide, Phosphorus oxybromide;Phosphoryl bromide
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Phosphorus oxybromide appears as a colorless crystalline solid or liquid if heated above 133 °F with a pungent odor. It is commonly heated and shipped in a molten state. Soluble in water, but, decomposed by water to hydrobromic and phosphoric acid with evolution of heat. Reacts with organic materials to cause fire. Evolves highly toxic and corrosive gases when exposed to fire. Corrosive to metals and tissue. Used to make other chemicals. |
|---|---|
| Color/Form | COLORLESS CRYSTALS |
| Boiling Point | AT 758 MM HG IT BOILS AT 193 °C WITH DECOMPOSITION |
| Melting Point | 133 °F (NOAA, 2007) |
| Solubility | SOL IN ETHER, BENZENE, CHLOROFORM, CARBON DISULFIDE, CONCENTRATED SULFURIC ACID |
| Density | greater than 1 (NOAA, 2007) |
| Decomposition | WHEN HEATED TO DECOMP, IT EMITS HIGHLY TOXIC FUMES OF BROMIDES & PO(X). |
| Other Experimental Properties | SLOWLY HYDROLYZES IN WATER FORMING PHOSPHORIC ACID & HYDROGEN BROMIDE; AT 758 MM HG IT BOILS AT 193 °C WITH DECOMPOSITION |
| Chemical Classes | Other Classes -> Halogens, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
COMPUTED DESCRIPTORS
| Molecular Weight | 286.69 g/mol |
|---|---|
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 285.72164 g/mol |
| Monoisotopic Mass | 283.72369 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 53 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Phosphorus oxybromide appears as a colorless crystalline solid or liquid if heated above 133 °F with a pungent odor. It is commonly heated and shipped in a molten state. Soluble in water, but, decomposed by water to hydrobromic and phosphoric acid with evolution of heat. Reacts with organic materials to cause fire. Evolves highly toxic and corrosive gases when exposed to fire. Corrosive to metals and tissue. Used to make other chemicals.