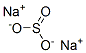CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium sulfite is a white odorless powder. Density 2.633 g / cm3. Moderately toxic. Sinks in water and dissolves slowly. Also transported as a heptahydrate Na2SO3.7H2O. |
|---|---|
| Color/Form | Small crystals or powder |
| Odor | Odorless |
| Taste | Saline, sulfurous taste |
| Boiling Point | Decomposes (NTP, 1992) |
| Melting Point | Decomposes (NTP, 1992) |
| Solubility | Soluble in 3.2 parts (NTP, 1992) |
| Density | 2.633 at 59 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Pressure | Negligible (NTP, 1992) |
| LogP | -4 |
| Stability/Shelf Life | It is fairly stable and does not oxidize as readily as hydrated sulfite. |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | When heated to decomposition it emits very toxic fumes of Na2O /sodium oxide/ and SOx /sulfur oxides/. |
| pH | 8,5-11,5 (anhydrous: 10 % solution; heptahydrate: 20 % solution) |
| Refractive Index | Index of refraction: 1.565 (powder), 1.515 (prism) |
| Other Experimental Properties | Density: 1.539. MP: decomposes; loses 7 H2O at 150 °C /Sodium sulfite heptahydrate/ |
| Chemical Classes | Other Classes -> Sulfites |
SAFETY INFORMATION
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P233:Keep container tightly closed. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304:IF INHALED: P312:Call a POISON CENTER or doctor/physician if you feel unwell. P321:Specific treatment (see … on this label). P340:Remove victim to fresh air and keep at rest in a position comfortable for breathing. P362:Take off contaminated clothing and wash before reuse. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P403:Store in a well-ventilated place. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 126.05 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 125.93635360 g/mol |
| Monoisotopic Mass | 125.93635360 g/mol |
| Topological Polar Surface Area | 82.4 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium sulfite is a white, granular or powdered solid. It is used in the pulp and paper industry, in the photographic industry to keep developer solutions from oxidizing and to wash fixer from film and photo-paper, in the textile industry as a bleach, desulfurizer or dechlorinator and in the tanning of leather.