7757-82-6
Product Name:
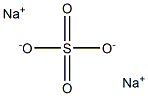
Sodium sulfate
Formula:
Na2SO4
Synonyms:
Disodium Sulfate;Natrii sulfas anhydricus;Sodium sulfate;Sodium Sulfate Anhydrous
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dry Powder; Dry Powder, Liquid, Other Solid; Dry Powder, Other Solid; Dry Powder, Pellets or Large Crystals; Dry Powder, Pellets or Large Crystals, Liquid; Dry Powder, Water or Solvent Wet Solid; Gas or Vapor; Liquid; Liquid, Other Solid; Other Solid; Pellets or Large Crystals; Water or Solvent Wet Solid |
|---|---|
| Color/Form | Powder or orthorhombic bipyramidal crystals |
| Odor | Odorless |
| Taste | Bitter saline taste |
| Melting Point | 884 °C |
| Solubility | In water, 28.1 g/100 g water at 25 °C |
| Density | 2.671 |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | When heated to decomposition it emits toxic fumes of sulfur oxides and sodium oxide. |
| Viscosity | 2.48 (22% solution at 20 °C) |
| Corrosivity | The rates of corrosion of iron and steel in water are a function of the specific mineral quality as well as the alkalinity and pH values. Sodium sulfate ... is a strong contributor to the rate of corrosion. For example, in water with 400 mg/l of alkalinity (as CaCO3) at pH 7, the corrosion rate will be zero at 200 mg/l of Na2SO4, but when the concentration of sodium sulfate is 400 mg/l, the corrosion rate will be about 100 mg per square cm per day. |
| pH | Neutral or slightly alkaline to litmus paper (5 % solution) |
| Refractive Index | 4.76 g/100 cc water at 0 °C, 42.7 g/100 cc water at 100 °C; soluble in glycerol; insoluble in alcohol; index of refraction: 1.484, 1.477, 1.471 /Natural thenardite/ |
| Dissociation Constants | Sodium sulphate dissociates into Na+ and SO4- ions in water. |
| Relative Evaporation Rate | Evaporation at 20 °C is negligible |
| Other Experimental Properties | Transition point to anhydrous form is 24.4 °C; white, rhombic or tetragonal; 19.5 g/100 cc water at 0 °C, 44 g/100 cc water at 20 °C/Sodium sulfate heptahydrate/ |
| Chemical Classes | Other Classes -> Sulfur Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 142.04 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 141.93126821 g/mol |
| Monoisotopic Mass | 141.93126821 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium sulfate is a white, odorless, and water-soluble powder that is used in a wide range of applications. It is often referred to as the mineral thenardite when found in its natural form.
RELATED SUPPLIERS
Panoli Intermediates (India) Pvt., Limited. (Kutch Chemical Industries Ltd.)
1Y
product:7757-82-6 Sodium sulfate 99%