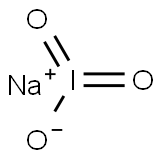CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White solid; [Hawley] White powder; ]MSDSonline] |
|---|---|
| Color/Form | White, crystal powder |
| Melting Point | MP: decomp |
| Solubility | Solubility in water: 9 g/100 cc @ 20 °C; 34 g/100 cc @ 100 °C; soluble in acetic acid; insol in alcohol |
| Density | 4.28 |
| Decomposition | When heated to decomposition it emits very toxic fumes. |
| Corrosivity | Neutral in solution |
| pH | Aq soln is neutral |
| Other Experimental Properties | Two hydrates exist in saturated solution, the pentahydrate up to 19.85 °C and the monohydrate from 19.85-73.4 °C. |
| Chemical Classes | Other Classes -> Halogens, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
COMPUTED DESCRIPTORS
| Molecular Weight | 197.892 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 197.87899 g/mol |
| Monoisotopic Mass | 197.87899 g/mol |
| Topological Polar Surface Area | 57.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 49.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium iodate is an organic molecular entity.