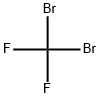
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dibromodifluoromethane is a colorless, nonflammable liquid. It may cause illness from ingestion and may be irritating to skin. If exposed to high temperatures it may emit toxic fumes. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Since it is a liquid it can easily penetrate the soil and contaminate groundwater and nearby streams. It is used as a fire extinguishing agent. |
|---|---|
| Color/Form | Colorless heavy liq |
| Odor | Characteristic odor |
| Boiling Point | 76 °F at 760 mmHg (NIOSH, 2023) |
| Melting Point | -231 °F (NIOSH, 2023) |
| Solubility | Insoluble (NIOSH, 2023) |
| Density | 2.29 at 59 °F (NIOSH, 2023) - Denser than water; will sink |
| Vapor Density | 7.2 (AIR= 1) |
| Vapor Pressure | 620 mmHg (NIOSH, 2023) |
| LogP | 1.99 |
| Decomposition | WHEN HEATED TO DECOMPOSITION, IT EMITS VERY TOXIC FUMES OF /HYDROGEN BROMIDE AND HYDROGEN FLUORIDE./ |
| Heat of Vaporization | 3.317X10+7 J/kmol @ -110 °C |
| Surface Tension | 3.976X10-2 N/m @ -110 °C |
| Ionization Potential | 11.07 eV |
| Refractive Index | Index of refraction: 1.399 @ 12 °C |
| Kovats Retention Index | 477 477 |
| Other Experimental Properties | 1 MG/L EQUIV TO 116.5 PPM & 1 PPM EQUIV TO 8.58 MG/CU M @ 25 °C, 760 MM HG |
| Chemical Classes | Solvents -> Chlorofluorocarbons |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H420:Hazardous to the ozone layer |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P502:Refer to manufacturer/supplier for information on recovery/recycling |
COMPUTED DESCRIPTORS
| Molecular Weight | 209.82 g/mol |
|---|---|
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 209.83143 g/mol |
| Monoisotopic Mass | 207.83348 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 30.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Dibromodifluoromethane is a colorless, nonflammable liquid. It may cause illness from ingestion and may be irritating to skin. If exposed to high temperatures it may emit toxic fumes. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Since it is a liquid it can easily penetrate the soil and contaminate groundwater and nearby streams. It is used as a fire extinguishing agent.