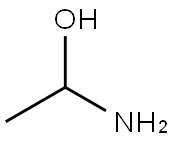
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Acetaldehyde ammonia appears as a white crystalline solid. Melting point 97 °C. Boiling point 110 °C (with some decomposition). An addition product between acetaldehyde and ammonia. Presents moderate fire and explosion hazard when exposed to heat or flame. Moderately toxic by ingestion and inhalation and a strong irritant. Used to make other chemicals, vulcanize rubber. |
|---|
COMPUTED DESCRIPTORS
| Molecular Weight | 61.08 g/mol |
|---|---|
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 61.052763847 g/mol |
| Monoisotopic Mass | 61.052763847 g/mol |
| Topological Polar Surface Area | 46.2 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 15.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Acetaldehyde ammonia appears as a white crystalline solid. Melting point 97 °C. Boiling point 110 °C (with some decomposition). An addition product between acetaldehyde and ammonia. Presents moderate fire and explosion hazard when exposed to heat or flame. Moderately toxic by ingestion and inhalation and a strong irritant. Used to make other chemicals, vulcanize rubber.