75-21-8
Product Name:
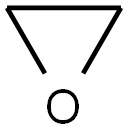
ETHYLENE OXIDE
Formula:
C2H4O
Synonyms:
Ethylene oxide solution;Oxirane
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ethylene oxide appears as a clear colorless gas with an ethereal odor with a flash point below 0 °F. Liquid less dense than water. Vapors heavier than air. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently. Vapors very toxic. Vapors irritate the eyes, skin, and respiratory system. Prolonged skin contact may result in delayed burns. Used to make other chemicals, as a fumigant and industrial sterilant. |
|---|---|
| Color/Form | Colorless ... gas at ordinary room temp and pressure; liquid below 12 °C |
| Odor | Sweet |
| Boiling Point | 51.3 °F at 760 mmHg (EPA, 1998) |
| Melting Point | -170.5 °F (EPA, 1998) |
| Flash Point | -0.4 to 0 °F (EPA, 1998) |
| Solubility | Miscible (NTP, 1992) |
| Density | 0.8222 at 50 °F (EPA, 1998) - Less dense than water; will float |
| Vapor Density | 1.49 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 1095 mmHg at 68 °F (EPA, 1998) |
| LogP | log Kow = -0.30 |
| Henry's Law Constant | Henry's Law constant = 1.48X10-4 atm-cu m/mol at 25 °C |
| Stability/Shelf Life | HYDROLYZES SLOWLY IN AQ SOLN |
| Autoignition Temperature | 804 °F (USCG, 1999) |
| Decomposition | Liquid ethylene oxide is not detonable, but the vapor may be readily initiated into explosive decomposition. |
| Viscosity | 9.45X10-3 mPa.s (25 °C, gas) and 0.254 mPa.s (10 °C, liquid) |
| Heat of Combustion | 1280.9 kJ/mol (liquid); 1306.1 kJ/mol (gas) |
| Heat of Vaporization | 24.75 kJ/mol at 25 °C |
| Ionization Potential | 10.56 eV |
| Polymerization | Precautions designed to prevent explosive polymerization of ethylene oxide are discussed, including rigid exclusion of acids, covalent halides such as aluminium, iron (III), and tin (IV) chloride, basic materials like alkali hydroxides, ammonia, amines, metallic potassium, and catalytically active solids such as aluminium or iron oxides or rust. |
| Odor Threshold | Odor Threshold Low: 257.0 [ppm] Odor Threshold High: 690.0 [ppm] Detection odor threshold from AIHA (mean = 420 ppm) |
| Refractive Index | Inex of refraction: 1.3597 at 7 °C/D |
| Kovats Retention Index | 417 417 405.3 405 400 405 400 |
| Other Experimental Properties | Heat of solution in water: 142.57 kJ/kg @ 25 °C |
| Chemical Classes | Pesticides -> Fumigants |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Gas Cylinder Compressed Gases GHS04  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H220:Flammable gases H280:Gases under pressure H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H340:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 44.05 g/mol |
|---|---|
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 44.026214747 g/mol |
| Monoisotopic Mass | 44.026214747 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 10.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ethylene oxide is a flammable gas with a somewhat sweet odor. It dissolves easily in water. Ethylene oxide is a man-made chemical that is used primarily to make ethylene glycol (a chemical used to make antifreeze and polyester). A small amount (less than 1%) is used to control insects in some stored agricultural products and a very small amount is used in hospitals to sterilize medical equipment and supplies.