71160-24-2
Product Name:
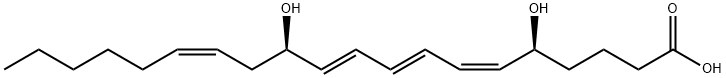
LEUKOTRIENE B4
Formula:
C20H32O4
Synonyms:
[5S,12R]-Dihydroxy-[6Z,8E,10E,14Z]-eicosatetraenoic acid;Leukotriene B₄ - CAS 71160-24-2 - Calbiochem;LTB₄, 5(S),12(R)-Dihydroxy-6- cis-8- trans-10- trans-14- cis-eicosatetraenoic Acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless liquid; [Fisher Scientific MSDS] |
|---|---|
| Collision Cross Section | 196.05 Ų [M+Na]+ [CCS Type: DT, Method: stepped-field] |
| Chemical Classes | Other Uses -> Biochemical Research |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 336.5 g/mol |
|---|---|
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 14 |
| Exact Mass | 336.23005950 g/mol |
| Monoisotopic Mass | 336.23005950 g/mol |
| Topological Polar Surface Area | 77.8 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 421 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Leukotriene B4 is a leukotriene composed of (6Z,8E,10E,14Z)-icosatetraenoic acid having (5S)- and (12R)-hydroxy substituents. It is a lipid mediator of inflammation that is generated from arachidonic acid via the 5-lipoxygenase pathway. It has a role as a human metabolite, a mouse metabolite, a plant metabolite and a vasoconstrictor agent. It is a dihydroxy monocarboxylic acid, a leukotriene, a long-chain fatty acid and a hydroxy polyunsaturated fatty acid. It is functionally related to an icosa-6,8,10,14-tetraenoic acid. It is a conjugate acid of a leukotriene B4(1-).